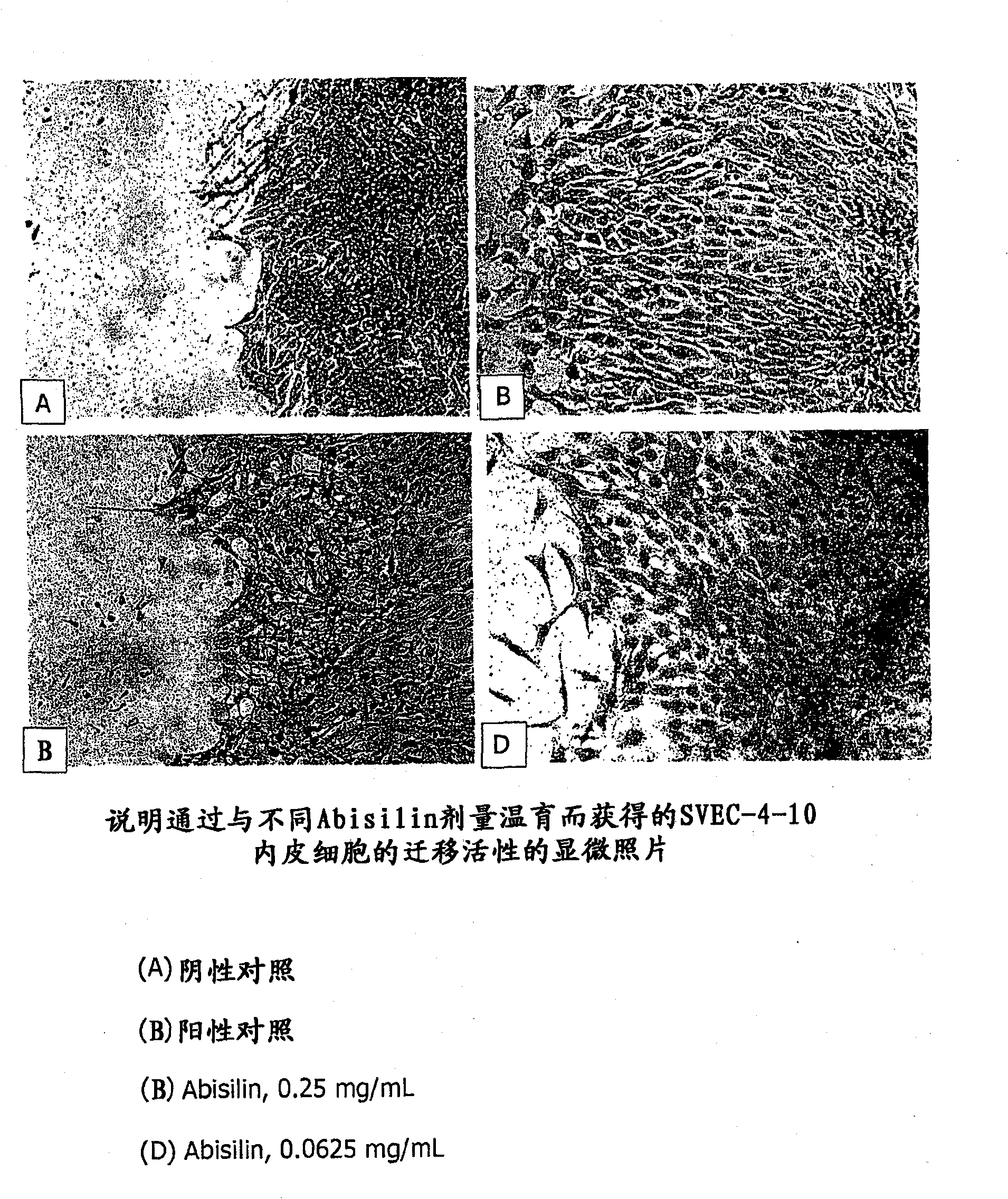
Antitumoral terpenoid pharmaceutical composition 'ABISILIN' exhibiting angiogenesis-inhibiting action
An angiogenesis and composition technology, which can be used in anti-tumor drugs, drug combinations, cardiovascular system diseases, etc., and can solve problems such as toxicity, loss of multiple activities of drugs, and low efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] In the determination of acute toxicity, no deaths of animals were observed at the maximum possible dose of 10000 mg / kg, which makes it possible to classify the drug in substance hazard category IV according to Russian state standard (GOST) 12.1.007-76 .
[0055] Subchronic experiments were performed on non-pedigree male and female rats with mean body weights of 165±3 grams and 163±3 grams, respectively. The study drugs were administered intragastrically once daily for 14 days at doses of 100, 500 or 1000 mg / kg for male rats and 100 and 1000 mg / kg for female rats.
[0056] According to the experimental results, the studied doses of Abisilin have significant effects on the overall indicators of experimental animals (weight gain, behavioral response, food and water intake); peripheral blood cell composition; liver excretion, absorption, protein synthesis and carbohydrate function; serum Cholesterol levels; and cardiovascular, excretory, and neurological functional status ...
Embodiment 2
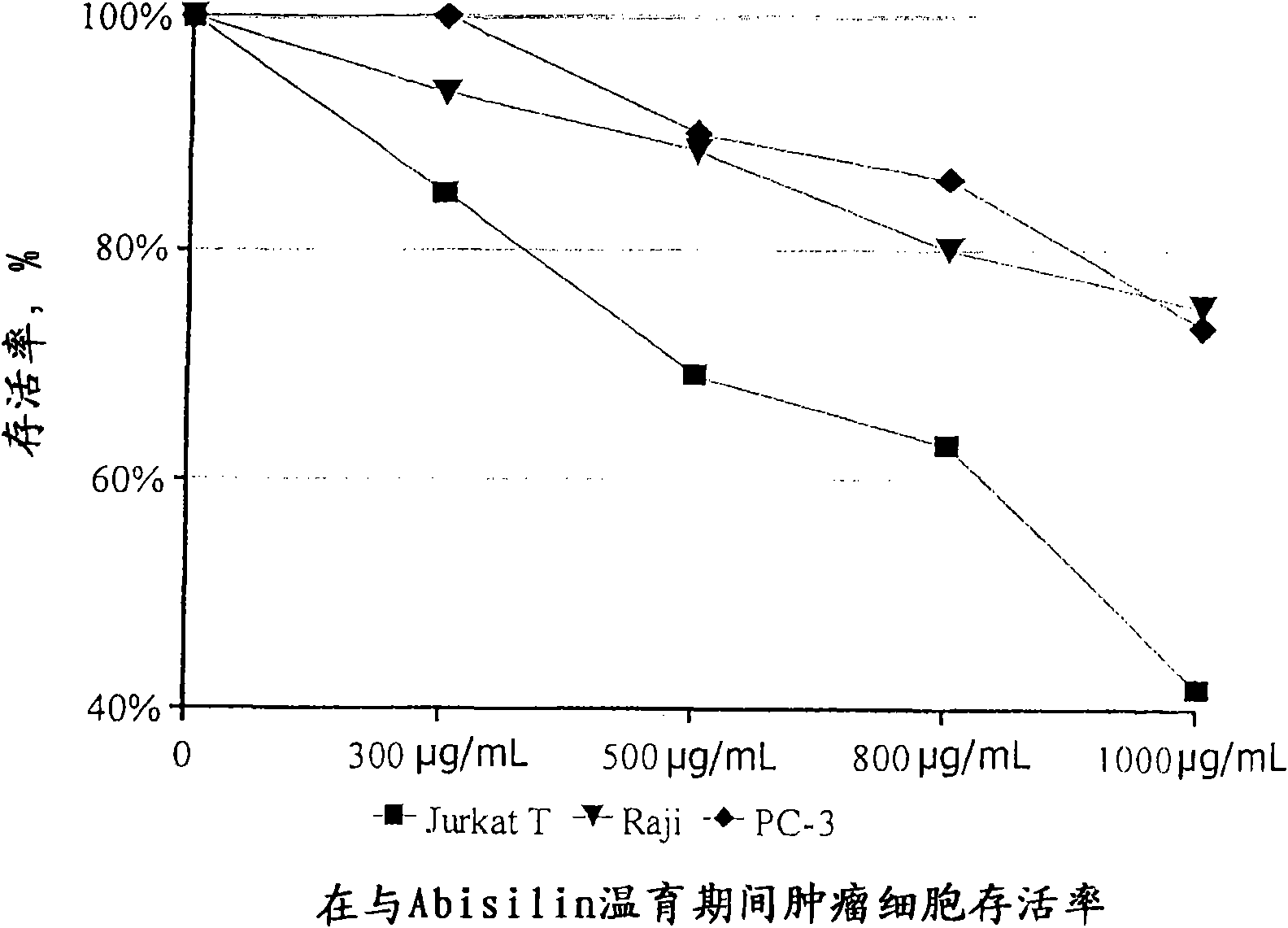
[0060] Cytotoxic and programmed cell death-inducing activities of Abisilin on tumor cells
[0061] In vitro studies were performed on the following tumor cell lines: Jurkat (T-cell lymphocytic leukemia), Raji (B-cell leukemia), K562 (chronic myeloid leukemia), U937 (myeloid leukemia), Mel P (melanoma) , T 47 D (breast cancer), SKOV-3 (ovarian cancer) and PC-3 (prostate cancer).
[0062] Incubate the above cell lines at 37°C and CO 2 Grow in complete RPMI-1640 medium containing 10% fetal bovine serum, 2 mM / mL glutamine, 0.1 mg / mL gentamicin, vitamins, sodium pyruvate, and amino acids in atmosphere.
[0063] In the study, Abisilin was used at the following concentrations: 300 μg / mL, 500 μg / mL, 800 μg / mL and 1000 μg / mL. 1% DMSO (dimethylsulfoxide) was used as diluent.
[0064] The cytotoxic activity of Abisilin was determined colorimetrically using the MTT assay. will be 5×10 4 Tumor cells at a concentration of 3 cells / ml were inoculated on a Saarsted flat-bottom 96-well pla...
Embodiment 3
[0083] Antitumor activity of oral pharmaceutical composition Abisilin in transplantable tumors
[0084] The antitumor activity of Abisilin was studied on mouse transplantable tumors included in the list of mandatory animal tumor models for screening new antitumor substances, namely: melanoma B-16, epidermoid Lewis lung cancer (LLC), breast cancer Ca-755, stage 5 cervical cancer CC-5, colon adenocarcinoma (CAC) and pleomorphic cell sarcoma M-1.
[0085] The experimental animals were BDF (C BI / 6×DBA / 2) and F (C D1 / 6 and CBA / 2) first-generation hybrid mice; CBA / 2, BALB / c and DBA / 2 strain mice (male and female), the body weight of each animal is 20-25 grams; and non-purebred pedigree female rats, the body weight of each animal is 200-250 grams. Mice and rats were purchased from the Experimental Animal Division of the Blokhin Russian Oncologic Center, the Russian Academy of Medicinal Sciences, and fed normal food rations.
[0086] Abisilin was administered orally (orally) as an o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com