Preparation method of yttrium-containing lithium titanate serving as cathode material of lithium ion secondary battery
A technology for secondary batteries and negative electrode materials, applied in battery electrodes, circuits, electrical components, etc., can solve problems such as poor discharge performance at high rates, and achieve the effect of improving electrical conductivity, excellent rate performance and cycle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
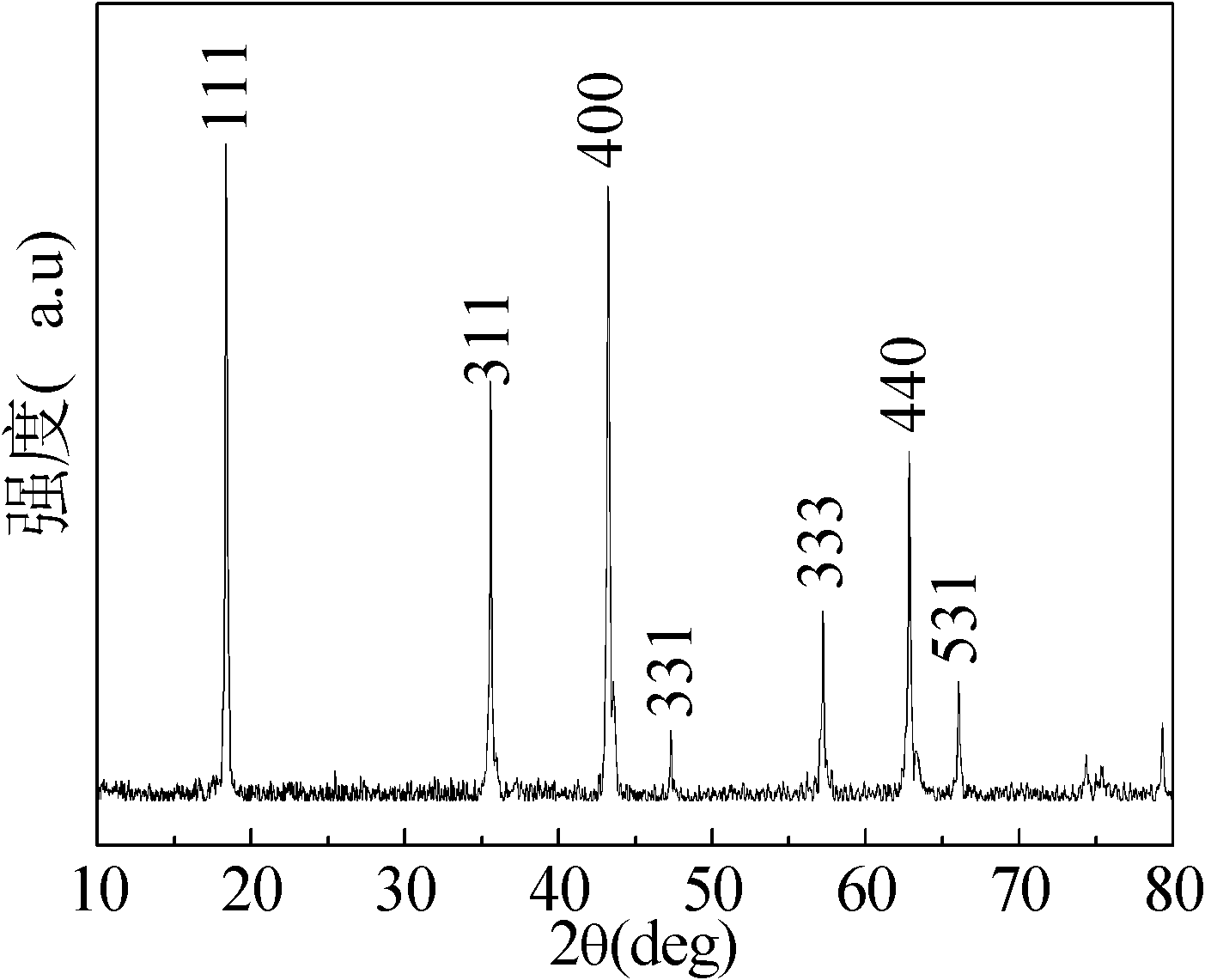
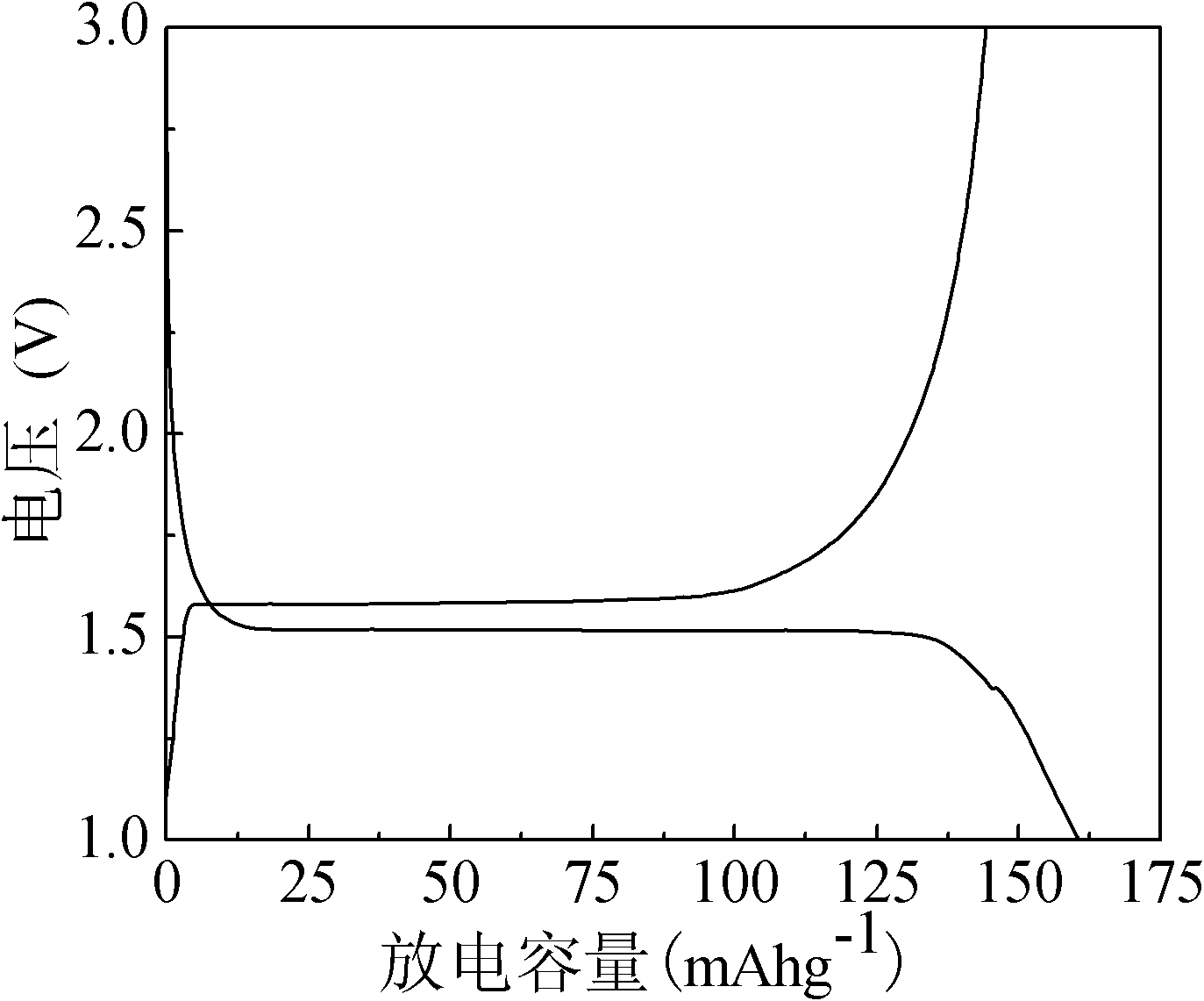
[0024] Synthesize 10 grams of Li 4 Y 0.1 Ti 4.9 o 12 , according to the molar ratio Li:Ti:Y=50:49:1, take by weighing 35.97 grams of tetrabutyl orthotitanate (analytical pure), 11.01 gram of lithium acetate (analytical pure) and 0.8266 gram of yttrium nitrate (analytical pure) ), 4.32 grams of lauric acid (analytically pure), which were dissolved in 8 milliliters of absolute ethanol respectively. Add the ethanol solution of lithium acetate dropwise to the ethanol solution of tetrabutyl orthotitanate, mix evenly under magnetic stirring, and react for 10 minutes, then add the ethanol solution of yttrium nitrate to the mixed solution, and wait for 10 minutes to react. Add an ethanol solution of lauric acid to the solution, and react for 25 hours at room temperature to obtain a uniform milky white gel. The gel was aged in the air for 24 hours, and then dried in an oven at 100° C. to obtain a pale yellow precursor. Put the precursor into a ball mill jar, add an appropriate amo...
Embodiment 2
[0030] Synthetic Li 4 Y 0.15 Ti 4.85 o 12 / C composite material, where Li 4 Y 0.15 Ti 4.85 o 12 The quality of is 10 grams, according to molar ratio Li:Ti:Y=80:97:3, takes by weighing 35.45 grams of tetrabutyl orthotitanate (analytical pure), 10.96 grams of lithium acetate (analytical pure) and 1.23 grams Yttrium nitrate (analytical pure), 3.65 g of citric acid (analytical pure), were dissolved in 10 ml of absolute ethanol. Add the ethanol solution of lithium acetate dropwise to the ethanol solution of tetrabutyl orthotitanate, mix evenly under magnetic stirring, and react for 15 minutes, then add the ethanol solution of yttrium nitrate to the mixed solution, wait for reaction for 15 minutes, and add Ethanol solution of citric acid was added to the solution, and reacted for 25 hours at room temperature to obtain a uniform milky white gel. The gel was aged in the air for 24 hours, and then dried in an oven at 120° C. to obtain a pale yellow precursor. Put the precursor...
Embodiment 3
[0032] Synthesize 10g of Li 4 Y 0.2 Ti 4.8 o 12, according to the molar ratio Li:Ti:Y=25:24:1, take by weighing 34.93 grams of tetrabutyl orthotitanate (analytical pure), 10.91 gram of lithium acetate (analytical pure) and 1.64 gram of yttrium nitrate (analytical pure) ), 1.56 grams of polyethylene glycol and 1.85 grams of citric acid (analytically pure), which were dissolved in 15 milliliters of absolute ethanol respectively. Add the ethanol solution of lithium acetate dropwise to the ethanol solution of tetrabutyl orthotitanate, mix evenly under magnetic stirring, and react for 10 minutes, then add the ethanol solution of yttrium nitrate to the mixed solution, and wait for 10 minutes to react. The ethanol solution of citric acid and polyethylene glycol was sequentially added into the solution, and reacted for 18 hours at room temperature to obtain a uniform milky white gel. The gel was aged in the air for 24 hours, and then dried in an oven at 100° C. to obtain a pale ye...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com