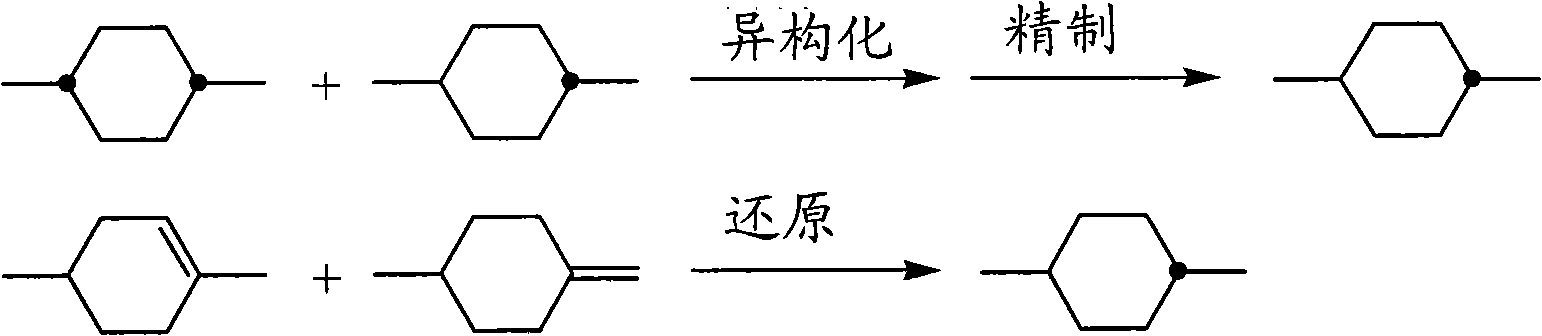
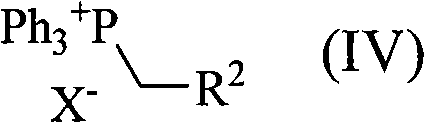Preparing method of compound with trans-1,4-cyclohexylidene structure
A manufacturing method and compound technology, applied in organic isomerization, chemical instruments and methods, organic chemistry, etc., can solve the problems of low selectivity between cis-isomers and trans-isomers, difficulty in obtaining trans-isomers, and inability to manufacture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] (Example 1) trans, trans-4-ethyl-4'-propyl bicyclohexane production
[0056] 【Reaction 1】
[0057]
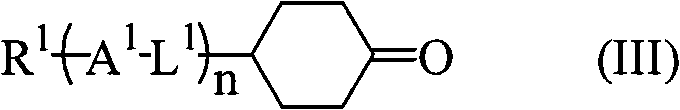
[0058] Add 400 g of ethyl triphenylphosphonium bromide, 200 g of 4-(trans-4'-propylcyclohexyl) cyclohexanone and tetrahydrofuran (THF) into a 2L 4-neck flask (with a mechanical stirrer and a thermometer). 500mL, ice-cooled. A THF solution (360 mL) of 122 g of potassium tert-butoxide was added dropwise thereto over 30 minutes, followed by stirring at 10° C. for 2 hours. Water was added, and after stirring, the organic layer was separated, and the aqueous layer was extracted with hexane. The organic layers were combined, washed with water, 50% methanol aqueous solution, and saturated brine, and the solvent was distilled off under reduced pressure, followed by purification to obtain 186 g of trans-4-ethylidene-4'-propylbicyclohexane.
[0059] 1 H-NMR (400MHz, CDCl 3 ): 0.85-1.15(m, 17H), 1.27-1.31(m, 3H), 1.54-1.76(m, 6H), 1.94-2.01(m, 1H), 2.15-2.21(m, 1H), 2.59-2....
Embodiment 2
[0065]In Example 1, the reaction solvent of the hydrogenation reaction was changed to tetrahydrofuran (THF), except that, the same operation was performed, and trans, trans-4-ethyl-4'-propylbicyclohexyl Production of alkanes. As in Example 1, the isomer ratio was measured by GC, and the trans, cis isomer was 15%, and the trans, trans isomer was 85%. Through the production method of the present invention, the trans-form and trans-isomers can be obtained with a high purity of 85%.
Embodiment 3
[0067] In Example 1, except that the reaction solvent of the hydrogenation reaction was changed to acetone, trans,trans-4-ethyl-4'-propylbicyclohexane was produced in the same manner. . As in Example 1, the isomer ratio was measured by GC, and the trans, cis isomer was 15%, and the trans, trans isomer was 85%. Through the production method of the present invention, the trans-form and trans-isomers can be obtained with a high purity of 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com