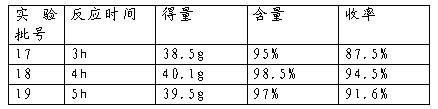
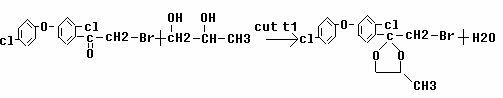Preparing and refining methods of difenoconazole
A technology of difenoconazole and its refining method, which is applied in organic chemistry and other fields, and can solve problems such as slow crystallization speed, incompletely dissolved impurities, unfavorable operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] (1) Preparation of Bromoketone
[0114] Put 0.1 mol of diphenyl ether and 0.105 mol of aluminum trichloride into a 2L reaction kettle equipped with a water separator and a condenser, respectively, and add 100 mol of dichloromethane. Cool to 15°C, add 0.105 mol of bromoacetyl chloride dropwise, finish dropping within 1 hour, keep warm for 4 hours, then add hydrochloric acid dropwise, remove the spent acid layer and the methylene chloride layer, desolventize, and use the methylene chloride to obtain bromoketone 35.5 g, content 96.3%.
[0115] (2) Preparation of brominated ketal
[0116] Slowly add 0.1 mol of bromoketone, 0.13 mol of propylene glycol, 1 g of cat, and 200 ml of toluene, heat at 110°C, and reflux for about 4 hours. g, content 95%, for the next step reaction.
[0117] (3) Synthesis and purification of difenoconazole
[0118] React 0.1 mol of brominated ketal, 0.13 mol of sodium triazole, 100 ml of xylene, and 1.0 g of catalyst (potassium iodide) at 120°C ...
Embodiment 2
[0120] (1) Preparation of Bromoketone
[0121] Put 0.15 mol of diphenyl ether and 0.158 mol of aluminum trichloride into a 2L reaction kettle equipped with a water separator and a condenser, respectively, and add 150 mol of dichloromethane. Cool to 12°C, add 0.158mol bromoacetyl chloride dropwise, finish dropping within 1h, keep warm for 4h, add hydrochloric acid dropwise, remove the spent acid layer and methylene chloride layer, desolventize, apply methylene chloride to obtain bromoketone 53.3g , content 96.5%.
[0122] (2) Preparation of brominated ketal
[0123] Slowly add 0.15mol of bromoketone, 0.19mol of propylene glycol, cat1.5g, and 300ml of toluene, heat at 105°C, and reflux for about 4 hours. After the reaction, cool to room temperature, remove the oil layer and toluene layer, and precipitate to obtain brominated ketal. Obtained 60.2g, content 95.5%. for the next reaction.
[0124] (3) Synthesis and purification of difenoconazole
[0125]React 0.15 mol of bromin...
Embodiment 3
[0127] (1) Preparation of Bromoketone
[0128] Put 0.2 mol of diphenyl ether and 0.21 mol of aluminum trichloride into a 2L reaction kettle equipped with a water separator and a condenser, respectively, and add 200 mol of dichloromethane. Cool to 10°C, add 0.21mol bromoacetyl chloride dropwise, finish dropping within 1 hour, keep warm for 4 hours, add hydrochloric acid dropwise, remove the spent acid layer and the methylene chloride layer, remove the solvent, and use the methylene chloride to obtain 71g of bromoketone. The content is 96.5%.
[0129] (2) Preparation of brominated ketal
[0130] Slowly add 0.2mol of bromoketone, 0.26mol of propylene glycol, cat2g, and 300ml of toluene, heat at 107°C, and reflux for about 4 hours. After the reaction, cool to room temperature, remove the oil layer and toluene layer, and desolventize to obtain brominated ketal, and obtain 80.2 g, content 96%. for the next reaction.
[0131] (3) Synthesis and purification of difenoconazole
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com