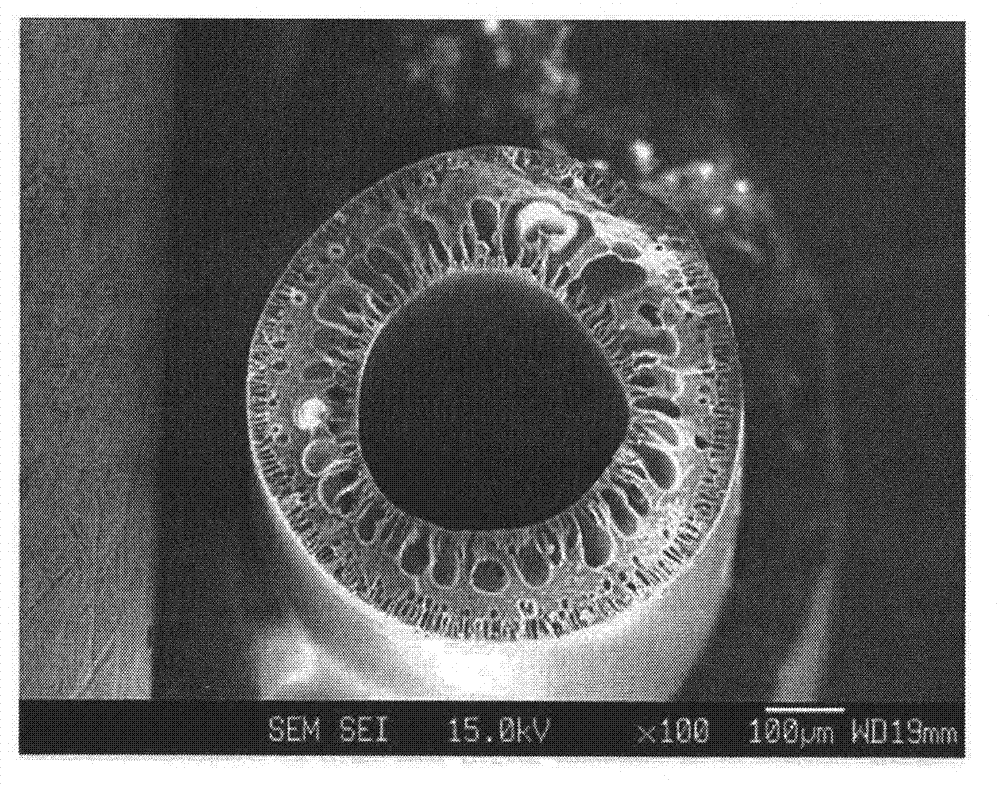
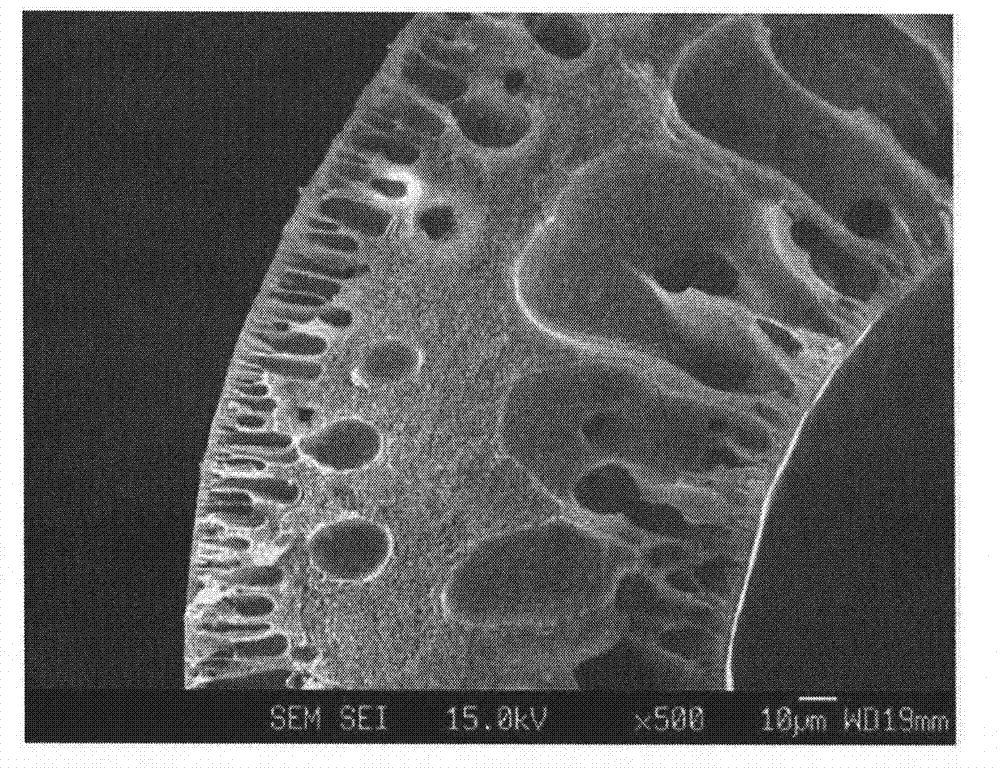
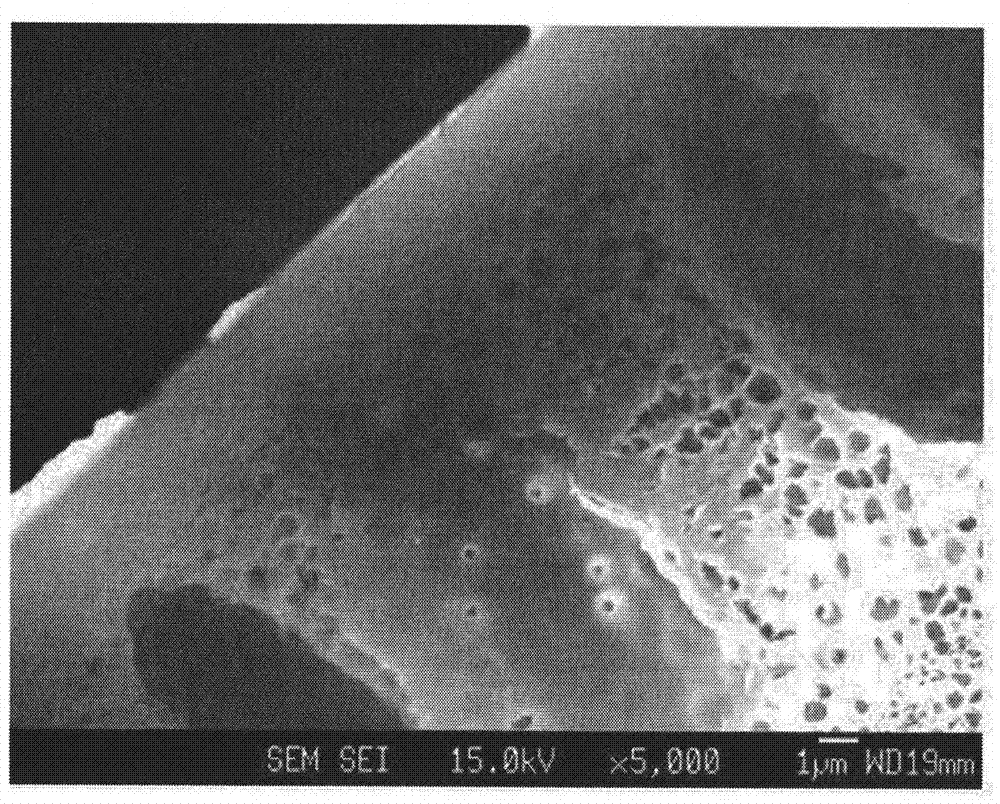
Hollow fiber, dope solution composition for forming a hollow fiber, and method for manufacturing a hollow fiber using the same
A fiber, hollow technology, applied in hollow filament manufacturing, chemical instruments and methods, single-component synthetic polymer rayon, etc., can solve the problems of membrane performance degradation, difficulty in obtaining separation ability and permeation ability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0412] Hollow fibers including polybenzoxazole represented by Chemical Formula 51 are prepared by the following reaction scheme 3 using a doping solution composition containing polyhydroxyamic acid for forming hollow fibers.
[0413] [Reaction Scheme 3]
[0414]
[0415] (1) Preparation of polyhydroxyamic acid
[0416] Add 36.6g (0.1mol) of 2,2-bis(3-amino-4-hydroxyphenyl)hexafluoropropane and 44.4g (0.1mol) of 4,4'-(hexafluoroisopropene) diphthalic anhydride Into 189 g (70 wt%) of N-methylpyrrolidone (NMP) and then reacted at 15°C for 4 hours to obtain a pale yellow viscous polyamic acid.
[0417] (2) Preparation of doping solution composition for forming hollow fibers
[0418] The obtained polyamic acid was added to 5 wt% of tetrahydrofuran as an additive without removing the solvent to prepare a uniform doping solution composition for forming hollow fibers.
[0419] (3) Preparation of hollow fiber
[0420] The doping solution composition for forming hollow fibers was defoamed under re...
Embodiment 2
[0424] The hollow fiber including the polybenzothiazole represented by Chemical Formula 52 is prepared by the following reaction using a doping solution composition containing polythioamic acid for forming hollow fibers.
[0425] [Chemical formula 52]
[0426]
[0427] The hollow fiber including the polybenzothiazole represented by the above Chemical Formula 52 was prepared in the same manner as in Example 1, except for the starting material, 20.8 g (0.1 mol) of 2,5-diamino-1,4- Dithiophene dihydrochloride and 44.4 g (0.1 mol) of 4,4'-(hexafluoroisopropylene) diphthalic anhydride were reacted to prepare a polyamic acid containing a thiol group (-SH).
[0428] The hollow fiber thus prepared has a weight average molecular weight of 14,500. As a result of FT-IR analysis, polybenzothiazole is at 1484cm -1 (C-S) and 1404cm -1 The characteristic frequency band of (C-S) was not detected in polyimide. The hollow fiber has a fractional free volume of 0.26 and interplanar spacing (d-spacing)...
Embodiment 3
[0431] The hollow fiber including the polypyrrolidone represented by Chemical Formula 53 is prepared by the following reaction using a doping solution composition containing polyaminoamic acid for forming hollow fibers.
[0432] [Chemical formula 53]
[0433]
[0434] The hollow fiber including the polypyridine represented by the above Chemical Formula 53 was prepared in the same manner as in Example 1, except for the starting materials, 21.4 g (0.1 mol) of 3,3′-diaminobenzidine and 44.4 g (0.1mol) of 4,4'-(hexafluoroisopropylene) diphthalic anhydride is reacted to prepare an amine group (-NH 2 ) Of polyamic acid.
[0435] The hollow fiber thus prepared has a weight average molecular weight of 18,000. As a result of FT-IR analysis, the polypyridine is at 1758cm -1 (C=O) and 1625cm -1 The characteristic band of (C=N) was not detected in polyimide. The hollow fiber has a fractional free volume of 0.28 and interplanar distance (d-spacing) of 630 pm.
[0436] The interplanar spacing (d-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| lattice spacing | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com