Preparation method of lithium iron phosphate and battery anode
A lithium iron phosphate, lithium source technology, applied in battery electrodes, chemical instruments and methods, circuits, etc., can solve the problems of cumbersome process, unsatisfactory conductivity, high cost, etc., and achieve simple process and high current charge and discharge performance. Excellent, low-cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
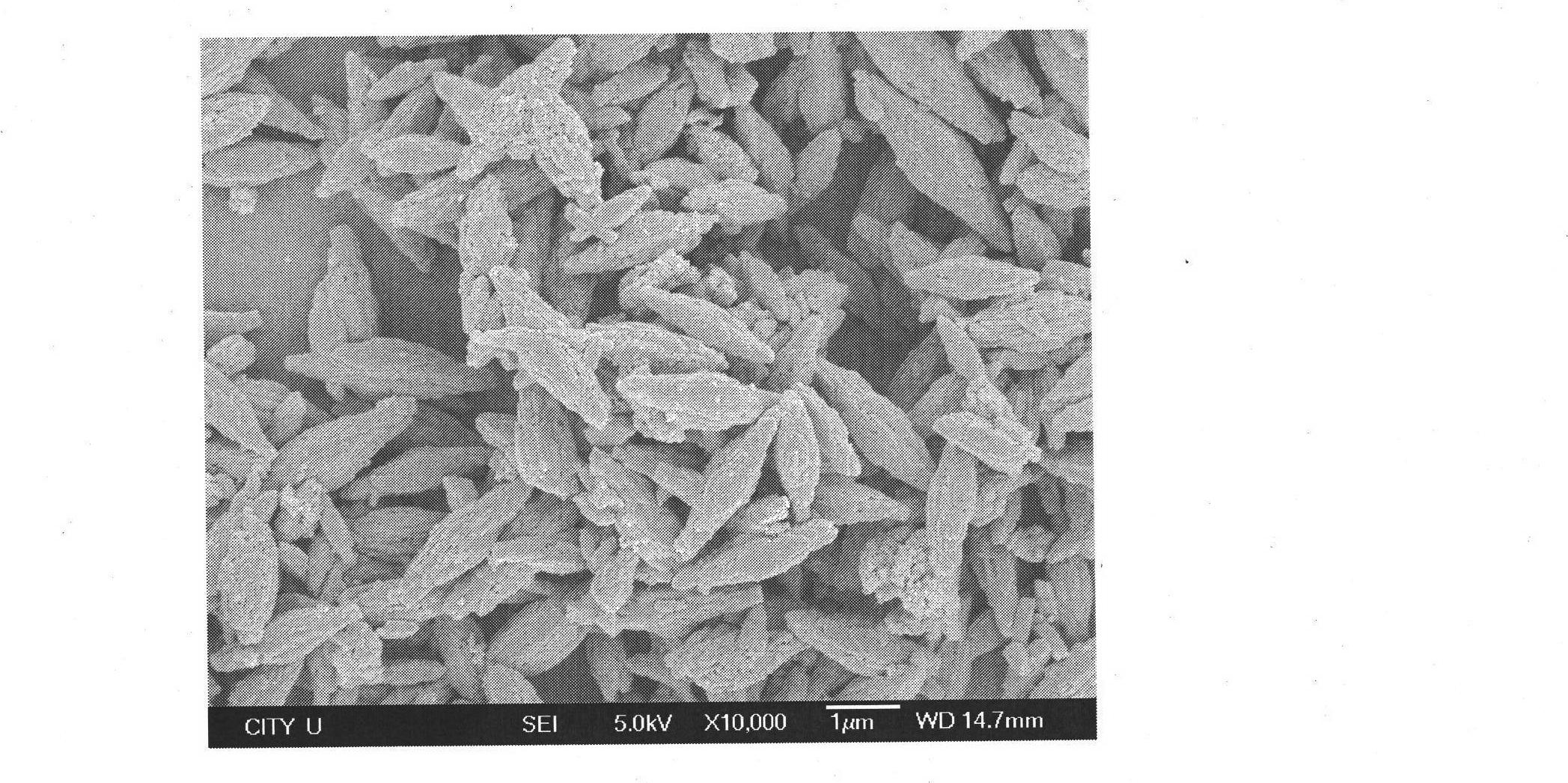
[0031] 0.01mol of ferrous acetate, 0.01mol of phosphoric acid and 0.02mol of LiOH·H 2 O were dissolved in 10ml of secondary deionized water under stirring, then the three transparent solutions were mixed to obtain a light green mixed precipitate, then 1g of PVP was added, the pH value of the mixed solution was adjusted to about 7 with urea aqueous solution, and the secondary deionized solution was added Ionized water to 100ml, stirred evenly, then transferred the mixture into a hydrothermal reaction vessel, screwed it tightly and placed it in a blast drying oven with a constant temperature of 180°C, reacted continuously for 12 hours, and then took the hydrothermal reaction vessel out of the oven, Place it in the air to cool down to room temperature naturally, filter, wash, and vacuum-dry at 30°C. The obtained material was analyzed by XRD to be phase-pure olivine LiFePO 4 structure, the space group is Pnma. SEM( figure 2 ) analysis shows that the product is a very uniform s...
Embodiment 3
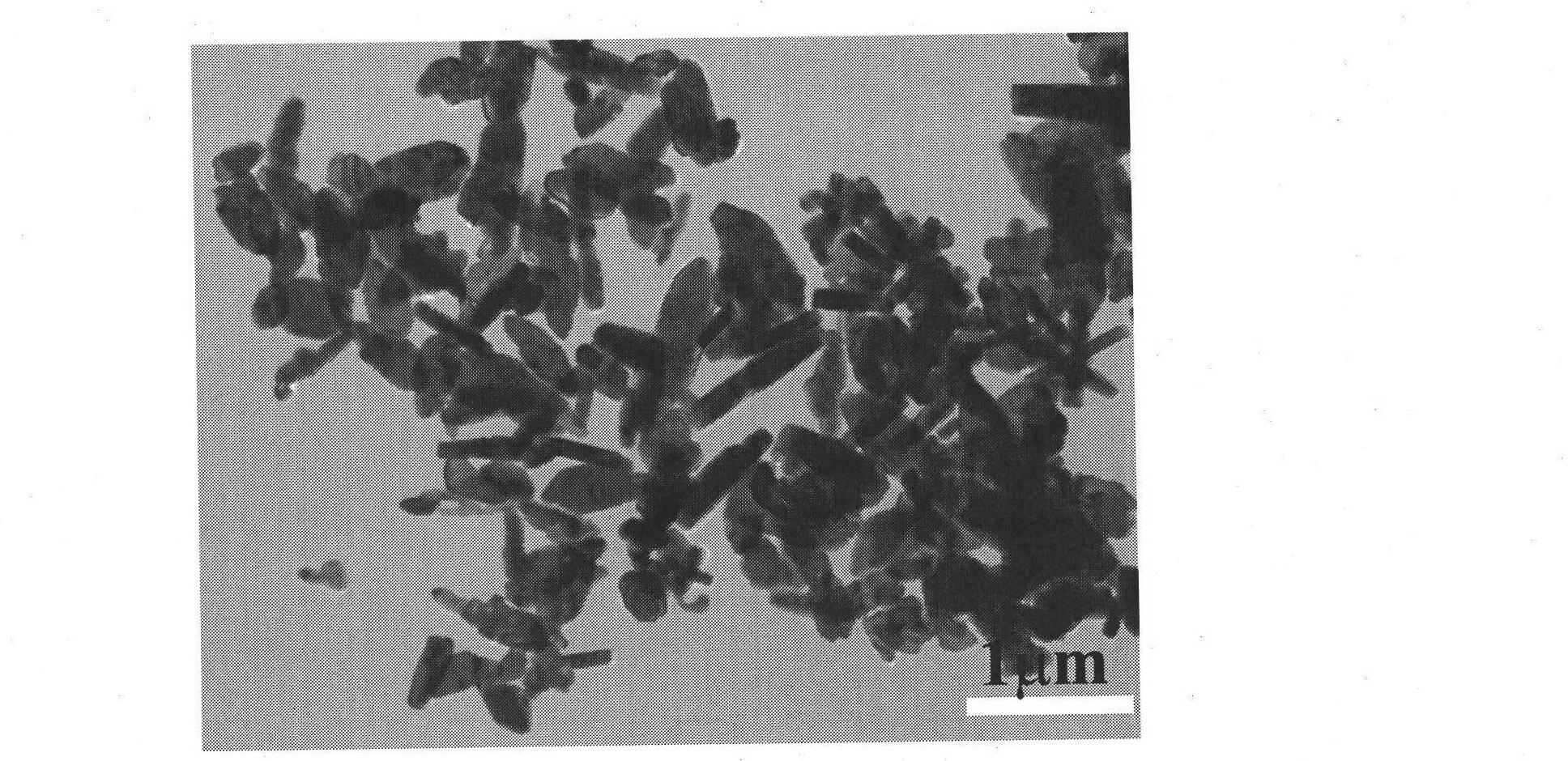
[0033] Dissolve 0.01mol of ferrous chloride, 0.01mol of dipotassium hydrogen phosphate and 0.03mol of lithium nitrate in 10ml of secondary deionized water respectively under stirring, then mix the three transparent solutions to obtain a light green mixed precipitate, Then add 20ml of ethylene glycol, adjust the pH value of the mixed solution to about 9 with NaOH aqueous solution, add secondary deionized water to 100ml, stir well, then transfer the mixture to a hydrothermal reaction vessel, screw it tightly and place it at a constant temperature of 200°C The reaction was continued for 24 hours in a blast drying oven, and then the hydrothermal reaction kettle was taken out of the oven, placed in the air to cool naturally to room temperature, filtered, washed, and vacuum-dried at 80°C. The obtained material was analyzed by XRD as pure phase olivine LiFePO 4 structure, the space group is Pnma. TEM( image 3 ) analysis shows that most of the product particles have the morphology ...
Embodiment 4
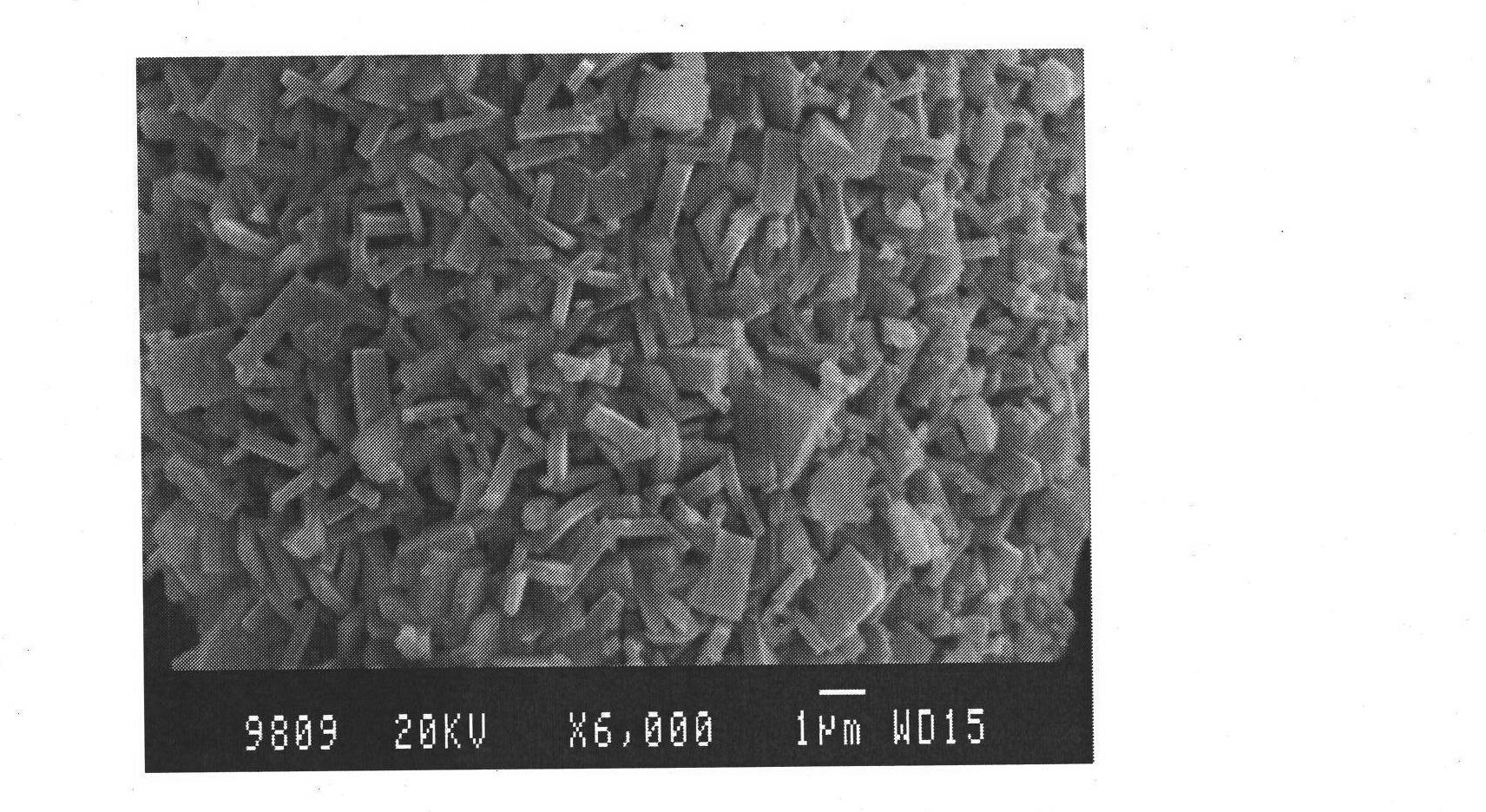
[0035] 0.01mol of ferrous nitrate, 0.01mol of phosphoric acid and 0.03mol of LiOH·H 2 O were dissolved in 10ml of secondary deionized water under stirring, and then the three transparent solutions were mixed to obtain a light green mixed precipitate, then 2g of citric acid was added, and the pH value of the mixture was adjusted to about 8 with ammonium carbonate, and then the secondary Deionized water to 100ml, stir evenly, then transfer the mixture to a hydrothermal reaction vessel, screw it tightly and place it in a blast drying oven with a constant temperature of 220°C, react continuously for 24 hours, and then take the hydrothermal reaction vessel out of the oven , placed in the air to cool naturally to room temperature, filtered, washed, and vacuum-dried at 60°C. The obtained material was analyzed by XRD as pure phase olivine LiFePO 4 structure, the space group is Pnma. SEM( Figure 4 ) analysis shows that the product is very uniform polyhedral micron particles with a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com