Method for decomposing vanadium slag by liquid phase oxidation
A technology of liquid-phase oxidation and vanadium slag, which is applied in the direction of improving process efficiency, etc., can solve the problems of chlorine-containing gas emission polluting the environment, chromium cannot be recycled at the same time, and vanadium recovery rate is low, so as to improve industrial operability and facilitate Industrial implementation, high utilization effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
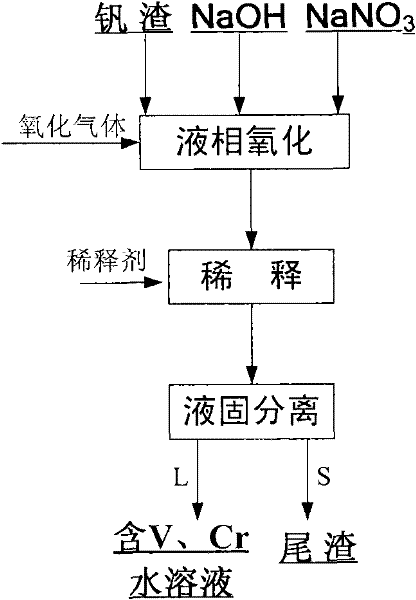
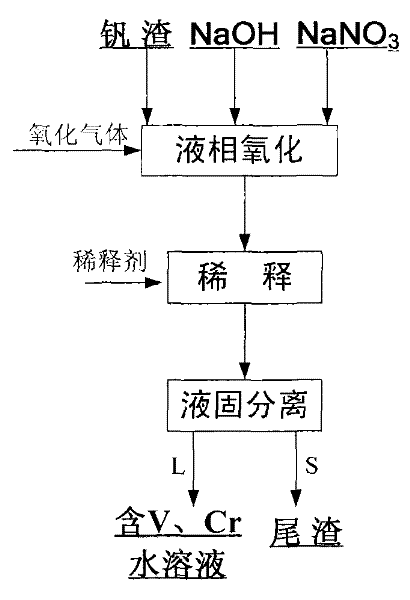
[0025] The vanadium slag used in this example is the vanadium (chromium) pig iron (water) produced from vanadium-titanium magnetite through the blast furnace process, and then the vanadium slag formed by the vanadium extraction process using air as the oxidation medium under high temperature conditions .
[0026] 1) First prepare the reaction materials: according to NaOH and NaNO 3 The mass ratio is 0.2:1, NaOH and NaNO 3 The mass ratio of the total amount to the vanadium slag is 6: 1 and weighed; 50g is weighed less than 200 mesh Chenggang vanadium slag (V in the vanadium slag 2 o 5 The content is 10.22wt%, Cr 2 o 3 Content is 3.98wt%), 50g NaOH and 250g NaNO 3 ;
[0027] 2) Liquid-phase oxidation reaction: NaOH and NaNO prepared in step 1) 3 Put it in a normal pressure reactor, set the reaction temperature to 420°C, start to heat up; rise to 420°C, add vanadium slag to NaOH-NaNO 3 In the molten salt, air was introduced, the air flow rate was controlled at 1L / min, and...
Embodiment 2
[0031] The vanadium slag used in this example is the vanadium-containing (chromium) pig iron (water) produced by the direct reduction process of vanadium-titanium magnetite, and then the vanadium formed by the process of extracting vanadium from molten iron with oxygen as the oxidation medium under high temperature conditions scum.
[0032] 1) First prepare the reaction materials: weigh 100g Chenggang vanadium slag (V in the vanadium slag 2 o 5 Content is 7.3wt%, Cr 2 o 3 content is 3.6wt%), 400g NaOH and 100g NaNO 3 , NaOH and NaNO 3 The mass ratio is 4:1, NaOH and NaNO 3 The mass ratio of the total amount to the vanadium slag is 5:1;
[0033] 2) Liquid-phase oxidation reaction: NaOH and NaNO prepared in step 1) 3 Place it in a normal pressure reactor, set the reaction temperature to 330°C, start to heat up; rise to 330°C, add vanadium slag to NaOH-NaNO 3 In the molten salt, feed oxygen, the flow rate of oxygen is controlled at 0.2L / min, and react for 0.5 hour under t...
Embodiment 3
[0037]The vanadium slag used in this example is the vanadium (chromium)-containing pig iron (water) produced from vanadium-titanium magnetite through the blast furnace process, and then is formed by the process of extracting vanadium in a top-blown re-blown converter under high temperature conditions with air as the oxidizing medium of vanadium slag.
[0038] 1) First prepare the reaction material: weigh 200g of Chenggang vanadium slag less than 200 mesh (V in the vanadium slag 2 o 5 The content is 10.22wt%, Cr 2 o 3 Content is 3.98wt%), 400g NaOH and 100g NaNO 3 , NaOH and NaNO 3 The mass ratio is 4:1, NaOH and NaNO 3 The mass ratio of the total amount to the vanadium slag is 2.5:1;
[0039] 2) Liquid-phase oxidation reaction: NaOH and NaNO prepared in step 1) 3 Place it in a normal pressure reactor, set the reaction temperature to 480°C, start to heat up; rise to 480°C, add vanadium slag to NaOH-NaNO 3 In the molten salt, a mixed gas of air and oxygen was introduced,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com