Content detection method of traditional Chinese medicine preparation solid-intestine capsule for treating chronic diarrhea
A technology for traditional Chinese medicine preparations and chronic diarrhea, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, and drug combinations, etc., can solve the problems of complexity, difficulty, and increased detection of index components, and achieve high accuracy and stable quality. , process stable and consistent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Recipe: Chishizhi (calcined) 500 parts Coptidis 250 parts Phellodendron 250 parts Myrobalan (pitted) 250 parts Nutmeg (steamed) 250 parts Magnolia officinalis (burned) 250 parts Jianqu 250 parts Evodia rutaecarpa (made) 200 parts Cinnamon 200 parts 250 parts of dried ginger 200 parts of Chinese prickly ash 200 parts of chuanxiong
[0027] Preparation method: mix the above fifteen crude drugs according to the ratio of the prescription amount, select to remove sundries, wash with water to remove sediment, dry, grind into fine powder, mix well, add appropriate amount of water, make granules, dry, and granulate , divided into capsules, that is. (From the authorized Guchang Capsule, patent number: ZL200510014980.1)
Embodiment 2
[0029] normal method
[0030] The content detection method of Guchang capsule, its method step is:
[0031] (1) Microscopic identification:
[0032] Take a small amount of the contents of Guchang Capsules and observe under a microscope: the bast fibers are bright yellow, bundled or scattered, and some are connected with stone cells, in the form of spindles or spindles, with oblique pointed ends, blunt rounded or narrow, 136 cm long ~185 μm, diameter 27~37 μm, wall thickness, lignification, pore grooves are obvious, parenchyma cells around fiber bundles contain square crystals of calcium oxalate, forming crystal fibers, square crystals are very dense, walls of cells containing crystals are unevenly lignified thickened.
[0033] ⑵ TLC identification of Chuanxiong:
[0034] Take 2 g of the contents of Guchang Capsules, add 10 ml of ethyl acetate, ultrasonically extract for 20 minutes, filter, and the filtrate is used as the test solution. Another 0.2 g of Chuanxiong referen...
Embodiment 3
[0048] Determination of Myrobalan and Galla by HPLC:
[0049] The prescription of Guchang Capsules contains gallnut and myrobalan, and its main component is tannin. Therefore, the method of determining gallic acid after hydrochloric acid hydrolysis is used to determine the content of tannin in the sample.
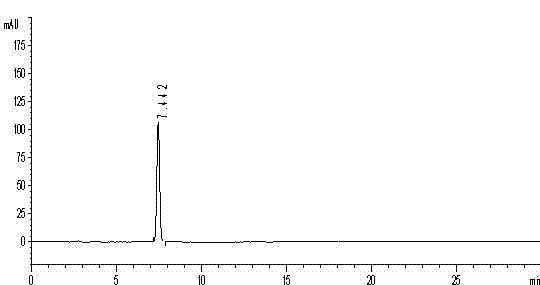
[0050] ①Preparation of reference substance solution: Take an appropriate amount of gallic acid reference substance, weigh it accurately, add 50% methanol to make a solution containing 40 μg of gallic acid per 1 ml, and obtain it. The liquid chromatography test results are shown in figure 1 .
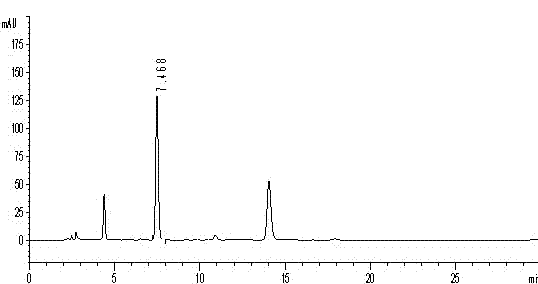
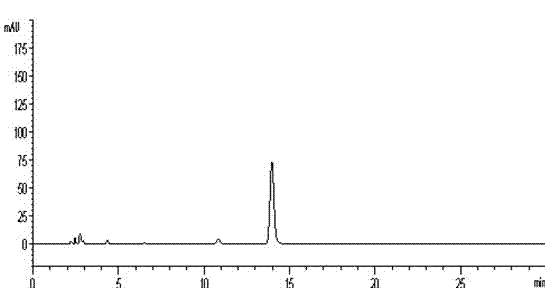
[0051] ②Preparation of the test solution: Take the content of Guchang Capsules under the item of difference in filling volume, mix well, grind finely, take 0.25g, accurately weigh, accurately add 50ml of 4mol / L hydrochloric acid, weigh, place in boiling water bath Heated and refluxed for 4 hours, let cool, weighed again, made up for the lost weight with 4mol / L hydrochloric acid, cen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com