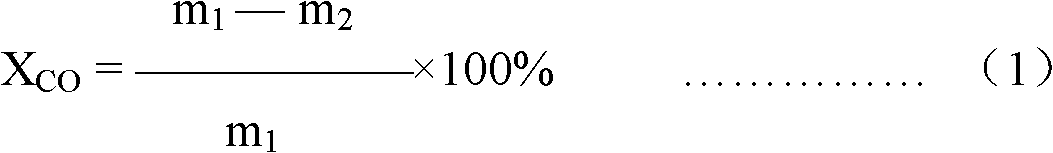Novel CO sulfur-tolerant shift catalyst and preparation method thereof
A sulfur-resistant shift, catalyst technology, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc. Change and other problems, to achieve the effect of good low sulfur activity, high activity and outstanding effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
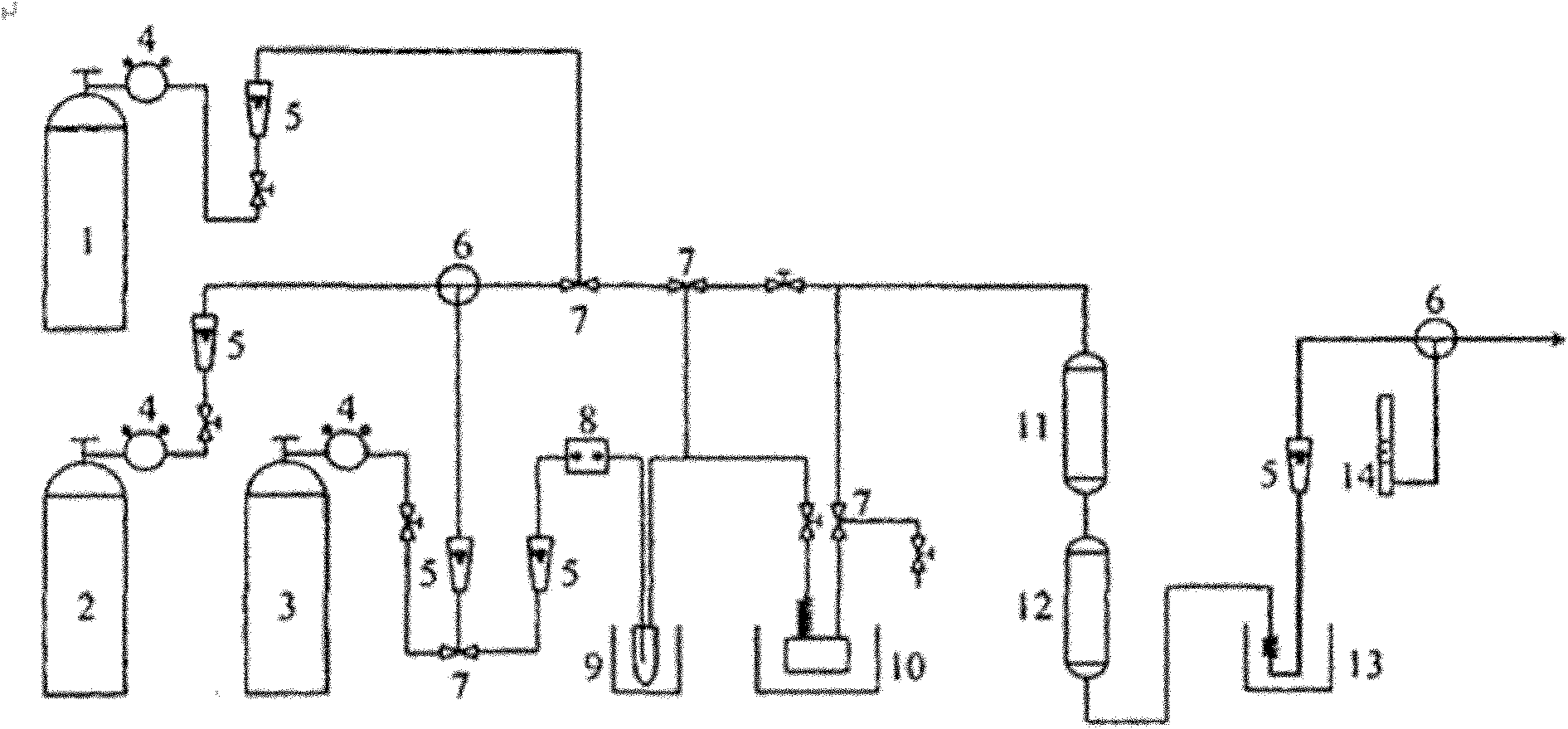
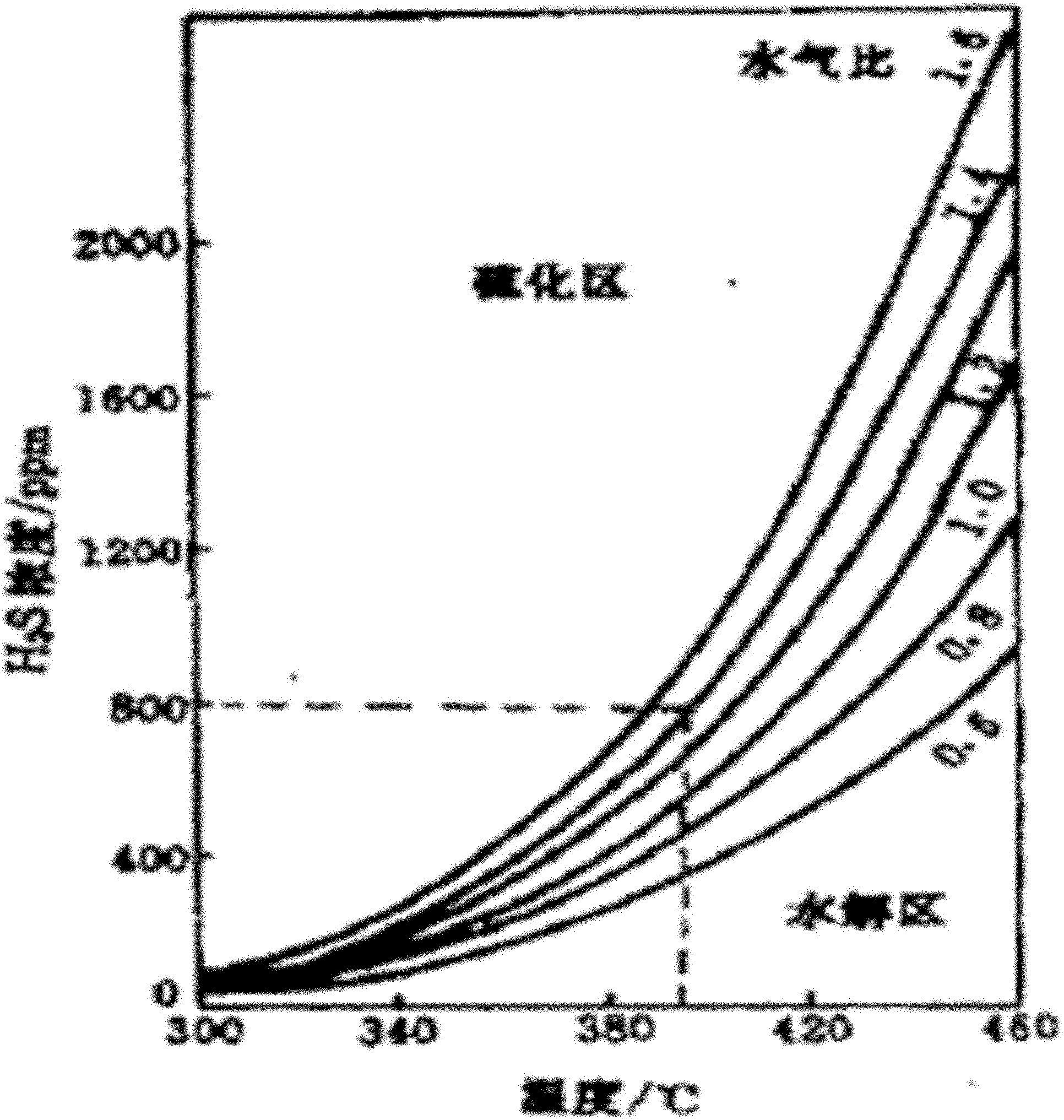
Image
Examples
Embodiment 1
[0073] Dry the industrial metatitanic acid at 120°C for 8 hours, pulverize it to 300 mesh, weigh 100 g of metatitanic acid and add it to the calcium nitrate aqueous solution (20 g Ca(NO) 3 ) 2 ·4H 2 (0 dissolved in 15g of water) after fully dipping and kneading, then adding 3g of concentrated sulfuric acid and 5g of polyacrylic acid amine and kneading for 2 hours, extruding and forming, the formed carrier was dried at 120 ° C for 10 hours, and under the condition of flowing air atmosphere, After calcination at 500°C for 3 hours, a Ca-modified titanium dioxide carrier was obtained.
[0074] 18.5g (NH 4 ) 6 Mo 7 O 24 ·4H 2 O, 10gCo(NO 3 ) 2 ·6H 2 O was successively added to the industrial ammonia solution, heated and stirred, and after all dissolved, a stable complex solution was prepared; the immersion solution was poured into 100 g of the above-mentioned Ca-modified titanium dioxide carrier, and after immersion for 1 hour, at 90 ~ 120 ° C It was dried for 8 hours and...
Embodiment 2
[0076] The industrial metatitanic acid was dried at 120°C for 8 hours, pulverized to 300 mesh, and 100 g of metatitanic acid was weighed into an aqueous solution of zinc nitrate and zirconium nitrate (22 g of Zn(NO) 3 ) 2 ·6H 2 O and 4gZr (NO 3 ) 4 .5H 2 (O dissolved in 25g of water) after fully immersing and kneading, then add 3g citric acid, 5g succulent powder and knead for 2 hours, then extrude and shape, the formed carrier is dried under superheated steam at 120 ° C for 4 hours, and then dried under flowing air. Under atmospheric conditions, calcination was carried out at 550°C for 2 hours to obtain a titanium dioxide carrier modified by Zn and Zr.
[0077] 12g (NH 4 ) 6 Mo 7 O 24 ·4H 2 O, 6gCo(NO 3 ) 2 ·6H 2 O, 4gNi (NO 3 ) 2 ·6H 2 O was successively added to the industrial ammonia solution, heated and stirred, and after all dissolved, a stable complex solution was prepared; the immersion solution was poured into 100 g of the above-mentioned Zn, Zr modifie...
Embodiment 3
[0079] The industrial metatitanic acid was dried at 120°C for 8 hours, pulverized to 300 mesh, and 100 g of metatitanic acid was weighed and added to the calcium nitrate aqueous solution (40 g Ca(NO) 3 ) 2 ·4H 2 (0 dissolved in 20 g of water) after fully immersing and kneading, then adding 2 g of concentrated sulfuric acid and 5 g of succulent powder and kneading for 2 hours, extruding and forming, and the formed carrier was dried under superheated steam at 120 ° C for 6 hours. Under the condition of air atmosphere, calcination at 400 °C for 4 hours to obtain a Ca-modified titanium dioxide carrier.
[0080] 16.0g (NH 4 ) 6 Mo 7 O 24 ·4H 2 O, 6.0gCo (NO 3 ) 2 ·6H 2 O, 2.5gNi (NO 3 ) 2 ·6H 2 O was successively added to the industrial ammonia solution, heated and stirred, and after all dissolved, a stable complex solution was prepared; the immersion solution was poured into 100 g of the above-mentioned Ca-modified titanium dioxide carrier, immersed for 1 hour, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com