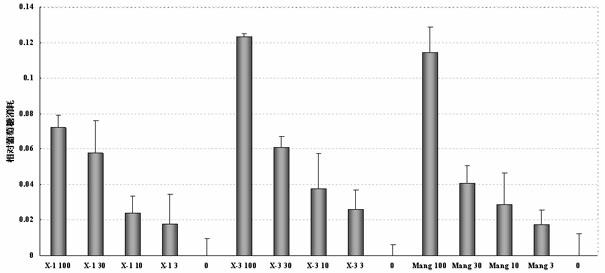
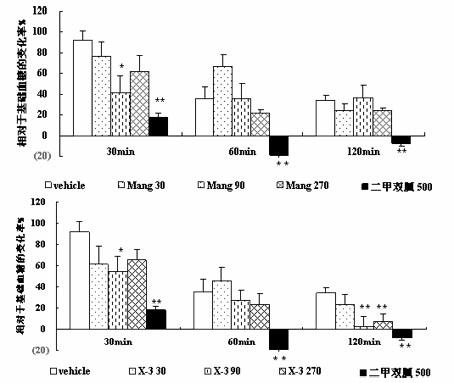
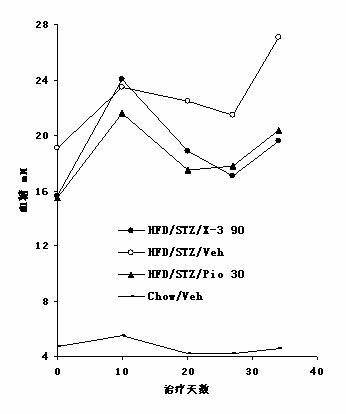
Mangiferin aglycon derivative, as well as preparation method and application of the mangiferin aglycon derivative
A mangiferin aglycone and derivative technology, applied in the field of compounds, can solve the problems of poor solubility and limitation, and achieve the effects of good development and utilization value, good reproducibility and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Preparation of 1,3,6,7-tetrahydroxy-xanthone (VI)
[0051] Please refer to the attached figure 1 , with figure 1 Is the preparation flow chart of 1,3,6,7-tetrahydroxy-xanthone.
[0052] (1) Preparation of 2,4,5-trimethoxybenzoyl chloride (II)
[0053] 25.00g (118mmol) of 2,4,5-trimethoxybenzoic acid was heated to reflux for 4 hours in the presence of 80mL of thionyl chloride to generate 26.80g of 2,4,5-trimethoxybenzoyl chloride (II), the yield 99%.
[0054] (2) Preparation of (2-hydroxy-4,5-dimethoxyphenyl)-(2,4,6-trimethoxyphenyl)-methanone (IV)
[0055] 26.80g (116.00mmol) of 2,4,5-trimethoxybenzoyl chloride and 20.00g (120.00mmol) of 1,3,5-trimethoxybenzene in 30.00g (220.00mmol) of anhydrous aluminum trichloride 30.40 g of (2-hydroxy-4,5-dimethoxyphenyl)-(2,4,6-trimethoxyphenyl)-methanone (IV) was produced at room temperature for 48 hours, with a yield of 75 %.
[0056] (3) Preparation of 1,3,6,7-tetramethoxy-xanthone (V)
[0057] (2-Hydroxy-4,5-dim...
Embodiment 2
[0060] Example 2 Preparation of target compound (X-1)
[0061] Please refer to the attached figure 2 , with figure 2 is a flow chart for the preparation of the target compound (X-1).
[0062] (1) Preparation of 1-hydroxy-3,6,7-tris(methoxymethoxy)-xanthone (VII)
[0063] 1,3,6,7-Tetrahydroxy-xanthone (VI) 2.60g (10.00mmol) in 4.1mL N,N - In the presence of diisopropyl, react with 2.50mL (31.00mmol) of chloromethyl methyl ether in an ice bath for 1h, the solvent is 150mL of dichloromethane, and then at room temperature for 8h, to obtain 1-hydroxyl-3,6,7-tri( Methoxymethoxy)-xanthone (VII) 2.20g, the pure product was obtained by column chromatography, and the yield was 55%.
[0064] (2) Preparation of compound (VIII)
[0065] 1-Hydroxy-3,6,7-tris(methoxymethoxy)-xanthone (VII) 2.20g (5.50mmol), add 8mL pyridine and 0.75g (8.30mmol) propane to 15mL acetone solution Acyl chloride was reacted under ice bath for 1.5 h to obtain 2.23 g of white solid (VIII), with a yield of ...
Embodiment 3
[0068] Example 3 Preparation of target compound (X-2)
[0069] At 0°C, dissolve VI 2.00g (5.40mmol) in 8mL pyridine and 10mL acetone solution, slowly add 1.25g (13.50mmol) propionyl chloride, and stir for 1.5h to obtain the target compound X-2 2.00g, please refer to attached image 3 , with image 3 Is the preparation flow diagram of the target compound (X-2), the yield is 70%. 1 H NMR (600MHz, DMSO) δ: 12.599 (1H, s, OH), 11.143 (1H, s, OH), 7.767 (1H, s, Ar-H), 6.971 (1H, s, Ar-H), 6.379 -6.382 (1H, d, J =1.8HZ, Ar-H), 6.211-6.214 (1H, d, J =1.8HZ, Ar-H), 2.571-2.643 (4H, m, 2CH 2 ), 1.139-1.263 (6H, m, 2CH 3 ); 13 C NMR(150MHz, DMSO) δ: 188.5, 169.1, 168.9, 162.5, 160.8, 160.3, 151.5, 143.2, 142.4, 123.5, 116.3, 111.7, 105.8, 96.3, 55.5, 60.0 MS, 9 (IES ) m / z calcd. for C 19 h 16 o 8 372.1, found [M-H] + 371.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com