Preparation method and application of perovskite type composite oxide catalyst
A composite oxide and perovskite-type technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve unfavorable scale production, many influencing factors, Problems such as high equipment cost, to achieve the effect of saving cumbersome procedures, simple process operation and low equipment cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
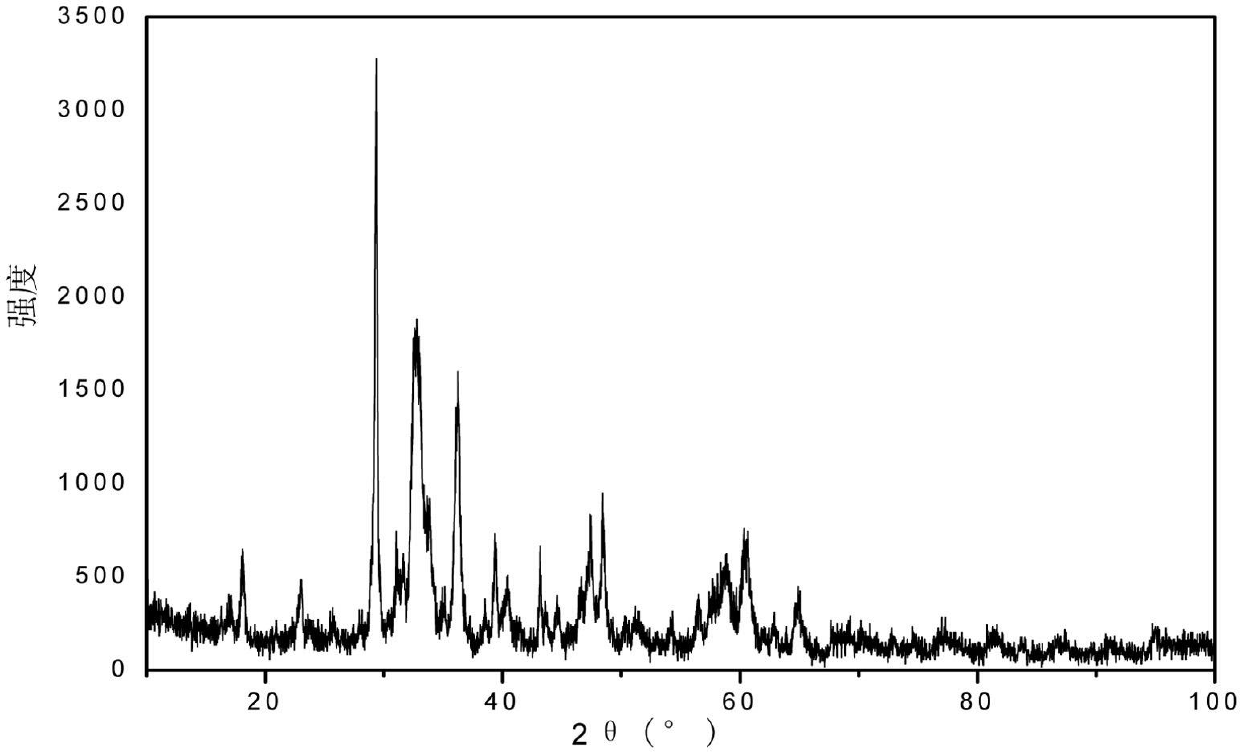
[0021] Select the constituent elements and chemical composition of the perovskite oxide, and according to the stoichiometric ratio, the selected three water-soluble salts: lanthanum nitrate (La(NO3)3 6H2O), calcium nitrate (Ca(NO3)2 4H2O) and manganese nitrate (50%Mn(NO3)2 solution) to form a solution with a total solute concentration of 5%, then weigh the coconut shell carbon mass of 3 / 4 of the total mass of the water-soluble salt added, and add it to the solution The activation solution was obtained, put it into an oven for drying at 80°C after standing for 10 hours, and then grind the obtained mixed powder, then send it to a muffle furnace for sintering at 700°C for 10 hours, and cool to room temperature with the furnace to obtain a perovskite type Composite oxide catalyst, the X-ray diffraction spectrum of gained oxide is as figure 1 As shown, the results show that the method can be used to prepare perovskite-type composite oxides.
Embodiment 2
[0023] According to the stoichiometric ratio, three selected water-soluble salts: lanthanum nitrate (La(NO 3 ) 3 ·6H 2 O), calcium nitrate (Ca(NO 3 ) 2 4H 2 O) and cobalt nitrate (Co(NO 3 ) 2 ·6H 2 O) Make a solution with a total solute concentration of 15%, then weigh XC-72 activated carbon that is 1 / 2 of the added water-soluble salt, add it to the solution to obtain an activation solution, put it into an oven at 85°C to dry after standing for 12 hours After drying, the obtained mixed powder was ground, then sent to a muffle furnace for sintering at 750° C. for 9 hours, and cooled to room temperature with the furnace to obtain a perovskite composite oxide catalyst.
Embodiment 3
[0025] According to the stoichiometric ratio, three selected water-soluble salts: lanthanum nitrate (La(NO 3 ) 3 ·6H 2 O), strontium nitrate (Sr(NO 3 ) 2 ) and manganese nitrate (50%Mn(NO 3 ) 2 solution) to make a solution with a concentration of 20% of the total solute, then weigh the fruit stone charcoal of the same quality as the added water-soluble salt, add it into the solution and stir to obtain an activation solution, and put it into an oven at 90°C to dry after standing for 8 hours. Then put the ground mixed powder into a muffle furnace for sintering at 800° C. for 8 hours, and cool down to room temperature with the furnace to obtain a perovskite composite oxide catalyst.
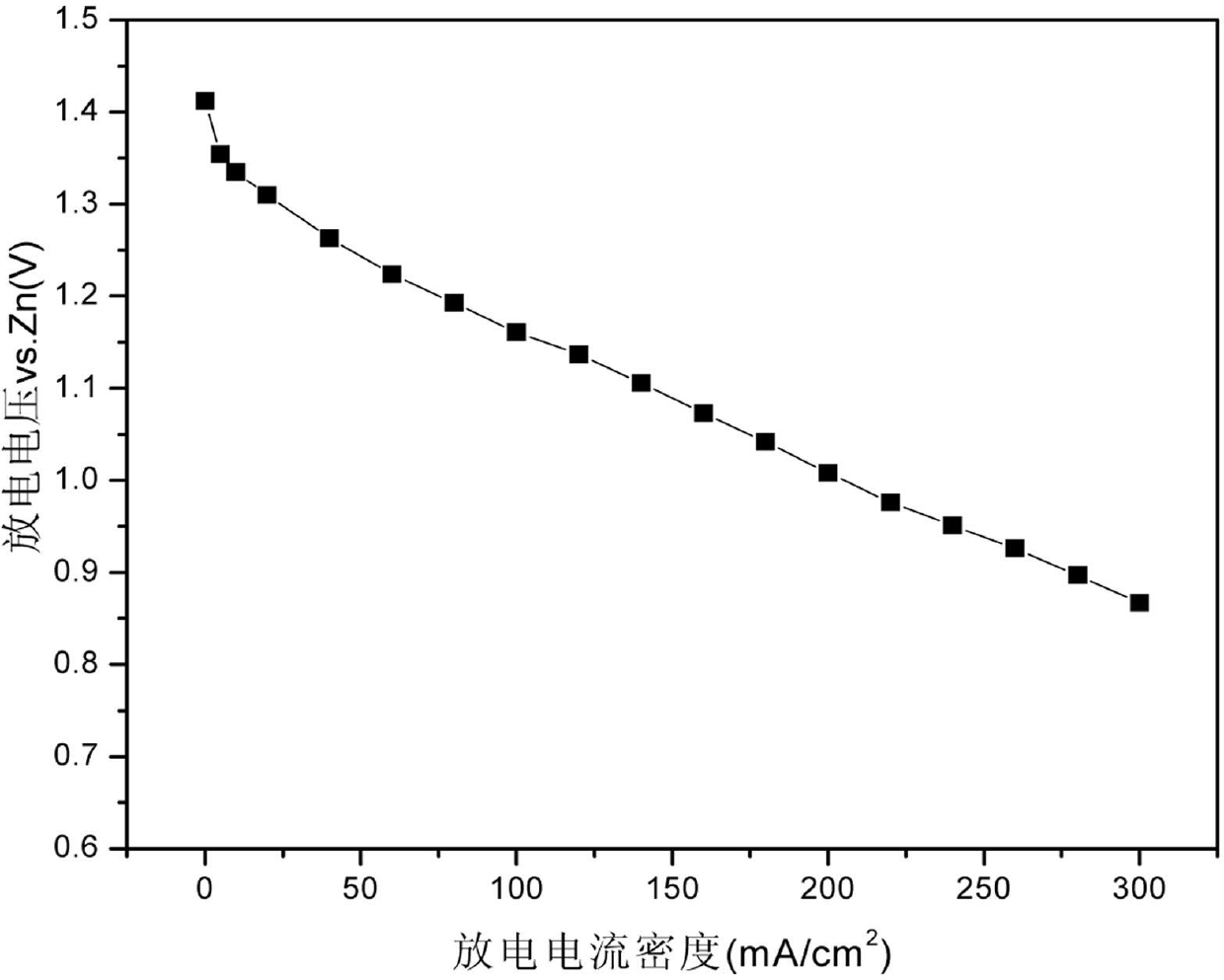
[0026] The use of the perovskite oxide catalyst prepared by the method of the present invention is preferably to prepare an air electrode.
[0027] The air electrode prepared by the catalyst of the present invention is synthesized by rolling a catalytic membrane, a hydrophobic membrane and a c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com