Method for synthesizing high-capacity solid hydrogen storage material ammonia borane by using amino complex
A technology of hydrogen storage materials and complexes, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, metal hydrides, etc., can solve high operating costs, low operating risks, and complex processes and other problems, to achieve the effect of mild preparation conditions, broaden the range of raw materials, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of hexammine nickel chloride:
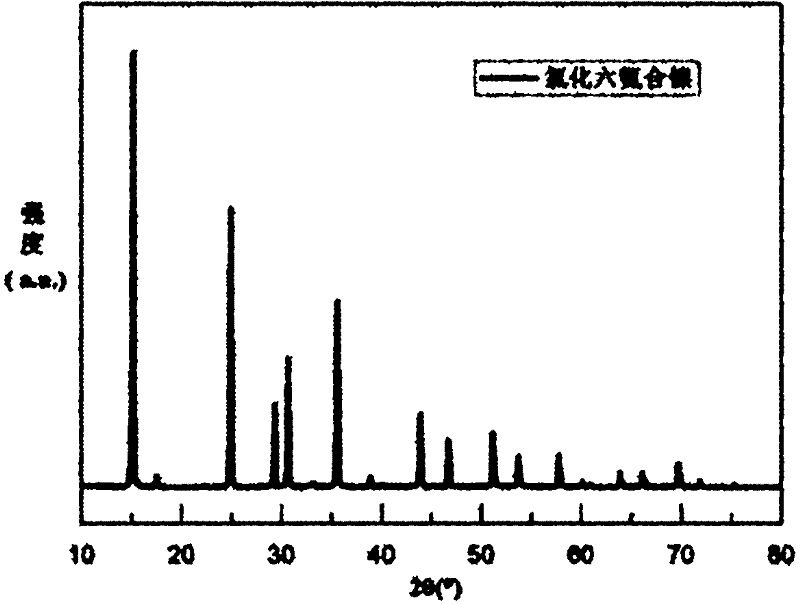
[0029] 18.6mmol NH 4 Cl was added to ammonia water, stirred and dissolved, and 5mmol Ni-Cl was slowly added to it 2 . 6H 2 O. Stirring was continued for 10 hours, and then the blue-purple solid was isolated by filtration, washed 2-3 times with absolute ethanol, and finally dried at 60° C. for 4 hours. The X-ray diffraction pattern shows that the prepared Ni(NH 3 ) 6 Cl 2 Corresponds exactly to the standard spectrum with good purity, such as figure 1 shown.
[0030] Preparation of high-capacity solid-state hydrogen storage material ammonia borane:
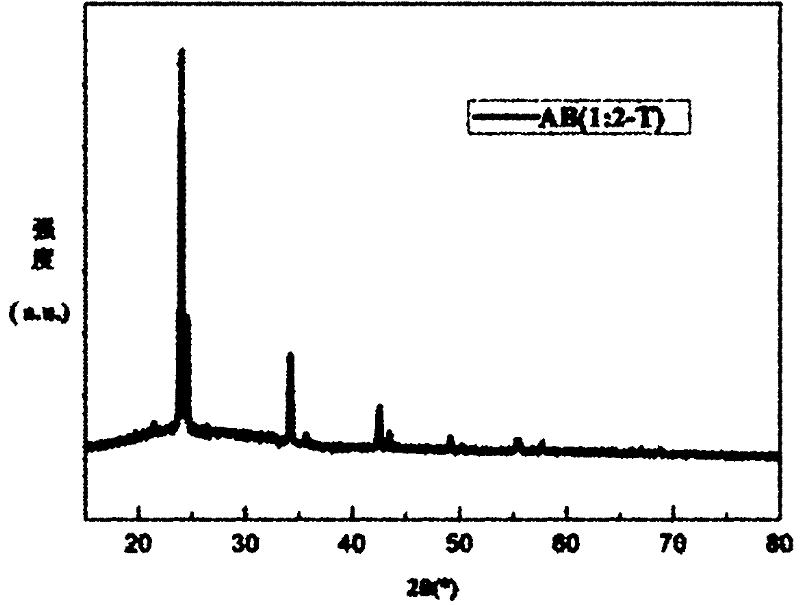
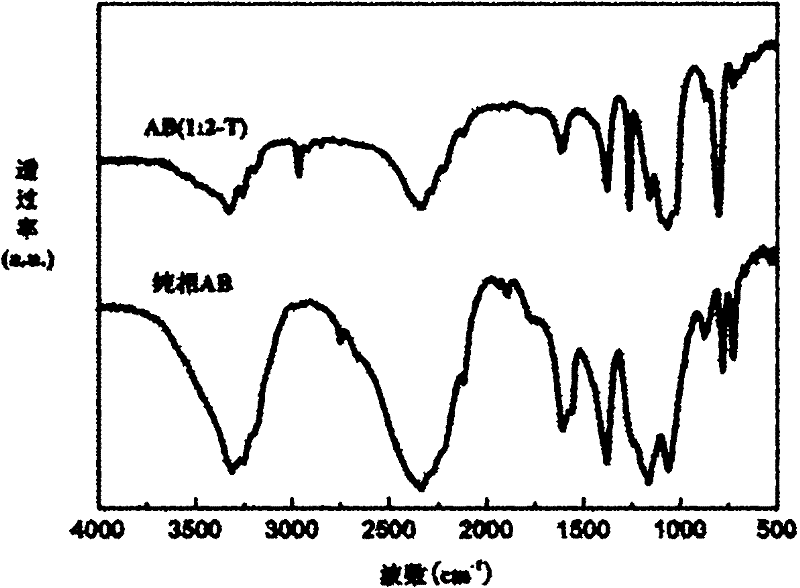
[0031] Add 0.64g hexaammine nickel chloride to the purified tetrahydrofuran solvent and stir thoroughly, then add 0.21g of sodium borohydride according to the molar ratio of hexaamine nickel chloride and sodium borohydride is 1:2, and continue stirring For 12 hours, the system maintained an argon atmosphere during the reaction. After centrifugation, the supernatant was ev...
Embodiment 2
[0037] The preparation of hexaammine nickel chloride is the same as in Example 1, after 0.64g hexaammine nickel chloride is added to the purified tetrahydrofuran solvent and fully stirred, then according to the hexaammine nickel chloride and sodium borohydride mol ratio is 1: Add 0.32 g of sodium borohydride in an amount of 3, and heat to reflux for 5 hours. During the reaction, the system maintains an argon atmosphere. After centrifugation, the supernatant was evaporated to obtain a white powder, and finally vacuum-dried at 40°C for 12 hours. The product was denoted as AB(1:3-H). Figure 7 For the X-ray diffraction pattern of ammonia borane prepared according to the above method, the pattern shows that there are no impurity peaks such as sodium borohydride and hexammine nickel chloride, and the purity is good.
Embodiment 3
[0039] The preparation of hexaammine nickel chloride is the same as in Example 1. After adding 0.64g hexaammine nickel chloride to the purified 1.4-dioxane solvent and fully stirring, then hexaammine nickel chloride and sodium borohydride Add 0.21 g of sodium borohydride at a molar ratio of 1:2. Insulated at 40° C. for 10 hours, and the system maintained an argon atmosphere during the reaction. The product was isolated, solvent removed, and dried to give the product, which was designated as AB(1:2-D). While maintaining a good purity of the product, the output is significantly increased.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com