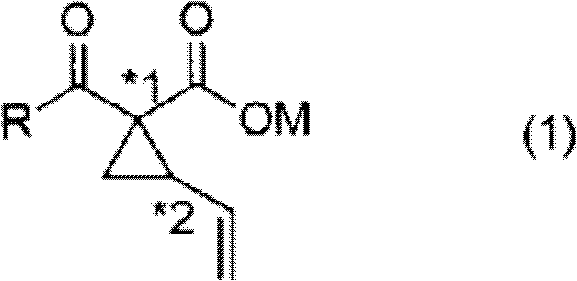
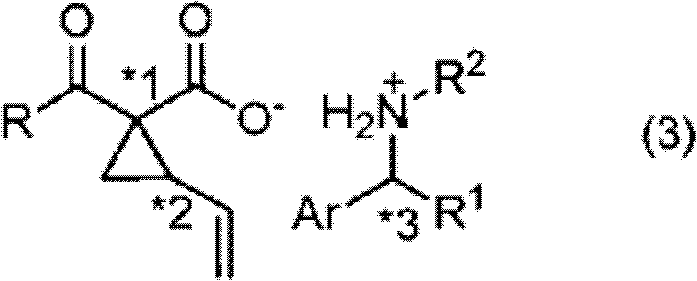
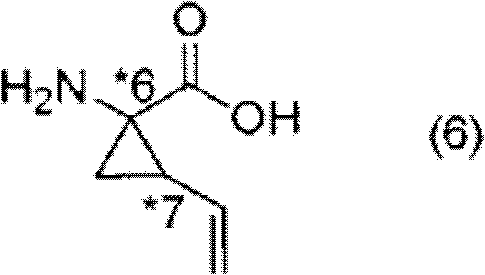
Optically active vinyl-cyclopropane carboxylic acid derivative and optically active vinyl-cyclopropane amino acid derivative manufacturing method
A technology of vinyl cyclopropane carboxylic acid and vinyl cyclopropane amide carboxylic acid, which is applied in the preparation of carbamic acid derivatives, carboxylic acid amide optical isomers, carboxylate esters, etc., and can solve the complex and efficient synthesis process. Low, low productivity and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
[0149] (Production Example 1) Production method of cis-2-vinyl-1-methoxycarbonylcyclopropanecarboxylic acid
[0150] While keeping the internal temperature at 1-5°C, 28 wt% sodium methoxide / methanol solution was added dropwise to trans-1,4-dibromo-2-butene (25.00 g, 0.144 mol), propane Dimethyl diacid (15.06 g, 0.144 mol) in methanol (150 mL). After completion of the dropwise addition, stirring was performed for 5 hours at an internal temperature of 24°C. Then, the reaction solution was cooled, and a 14% potassium hydroxide aqueous solution (36.02 g, 0.091 mol) was added dropwise over 30 minutes while maintaining the internal temperature at 1 to 5°C. After the dropwise addition, the mixture was stirred for another 21 hours at an internal temperature of 24° C., and then concentrated using a rotary evaporator until the total amount was about 70 g. To the concentrate were added water (45 mL), tert-butyl methyl ether (75 mL), and then concentrated hydrochloric acid until the pH...
manufacture example 2
[0151] (Production Example 2) trans-2-vinyl-1-carbamoylcyclopropanecarboxylic acid {(1S, 2S)-, (1R, 2R)- 1:1 mixture of 2-vinyl-1-carbamoylcyclopropanecarboxylic acid} manufacturing method
[0152] While keeping the internal temperature at 1-5°C, 28 wt% sodium methoxide / methanol solution was added dropwise to trans-1,4-dibromo-2-butene (25.00 g, 0.144 mol), propane Dimethyl diacid (15.06 g, 0.144 mol) in methanol (150 mL). After the dropwise addition, under the condition of an internal temperature of 24°C, stir for 5 hours, then, while maintaining the internal temperature at 15-25°C, introduce ammonia gas (about 15g, 0.88mol) into the reaction solution for 1 hour . Stirring was performed for 15 hours at an internal temperature of 25° C., and then concentrated using a rotary evaporator until the total amount was about 142 g. Water (17.44g) and 30 wt% sodium hydroxide aqueous solution (12.17g, 0.09mmol) were added to the concentrate, and it stirred for 4 hours on condition...
Embodiment 1
[0153] (Example 1) (1S, 2S)-2-vinyl-1-carbamoylcyclopropanecarboxylic acid (S)-N-benzyl-1-benzene The manufacture method of ethyl ethylamine salt
[0154] A 1:1 mixture of (1S, 2S)-type and (1R, 2R)-type synthesized according to Production Example 2, namely 2-vinyl-1-carbamoylcyclopropanecarboxylic acid (500 mg, 3.22 mmol) was suspended It was mixed in acetonitrile (5 mL), and (S)-N-benzyl-1-phenylethylamine (681 mg, 3.22 mmol) was added thereto at room temperature. Stirring was performed for 1 hour at the same temperature, and precipitated crystals were obtained by filtration. The mixture was washed with acetonitrile (1 mL) cooled to 0° C. and dried to obtain the title compound (yield: 416.9 mg, yield: 35.3%).
[0155] The optical purity of the obtained compound was measured by high-performance liquid chromatography (HPLC), and it was 95.6% ee.
[0156] (HPLC analysis system) column: CHIRALCEL OJ-H, eluent: hexane / isopropanol / trifluoroacetic acid=90 / 10 / 0.1, flow rate: 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com