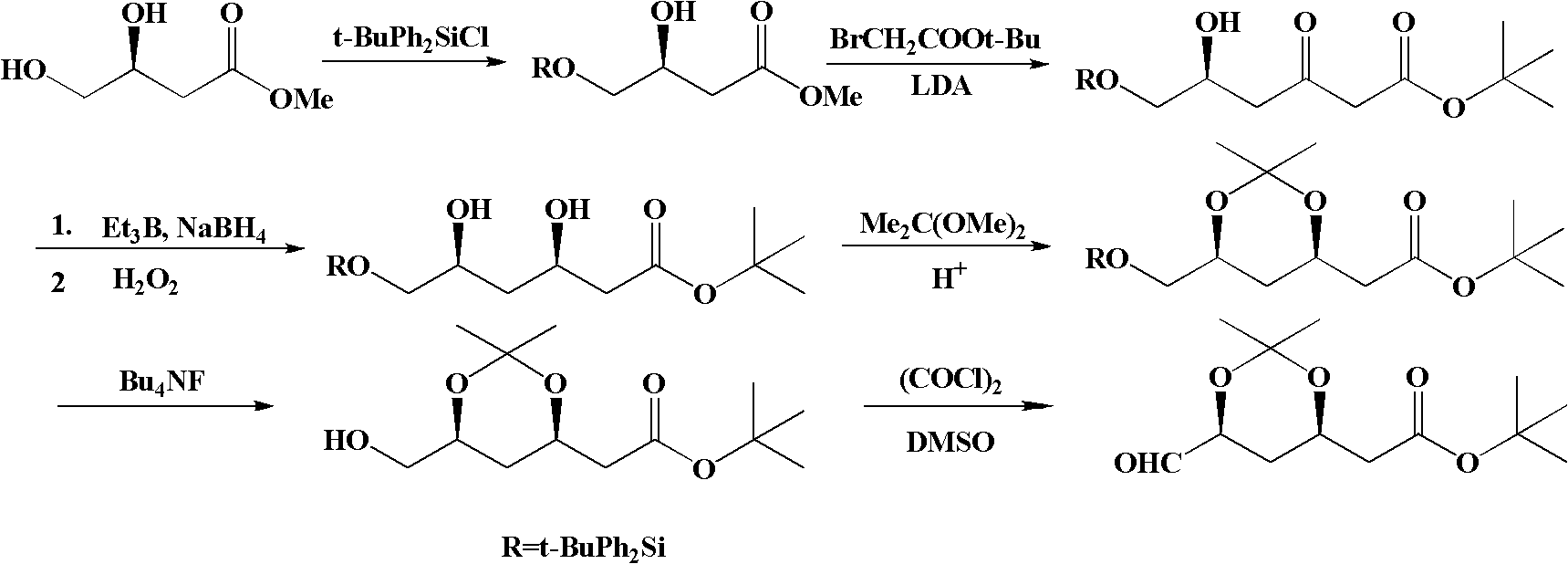
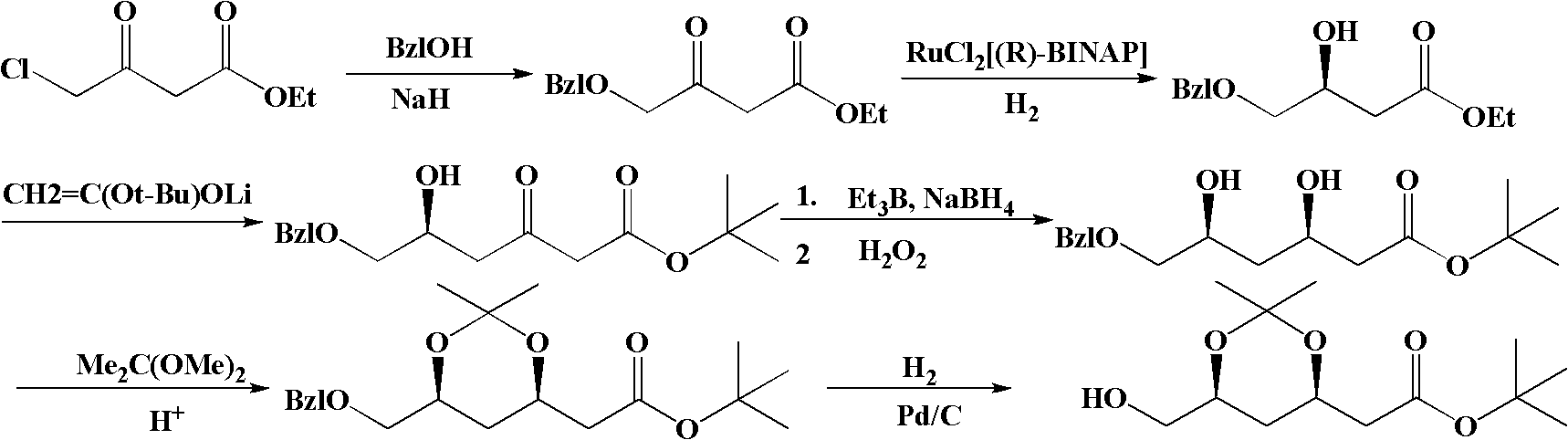
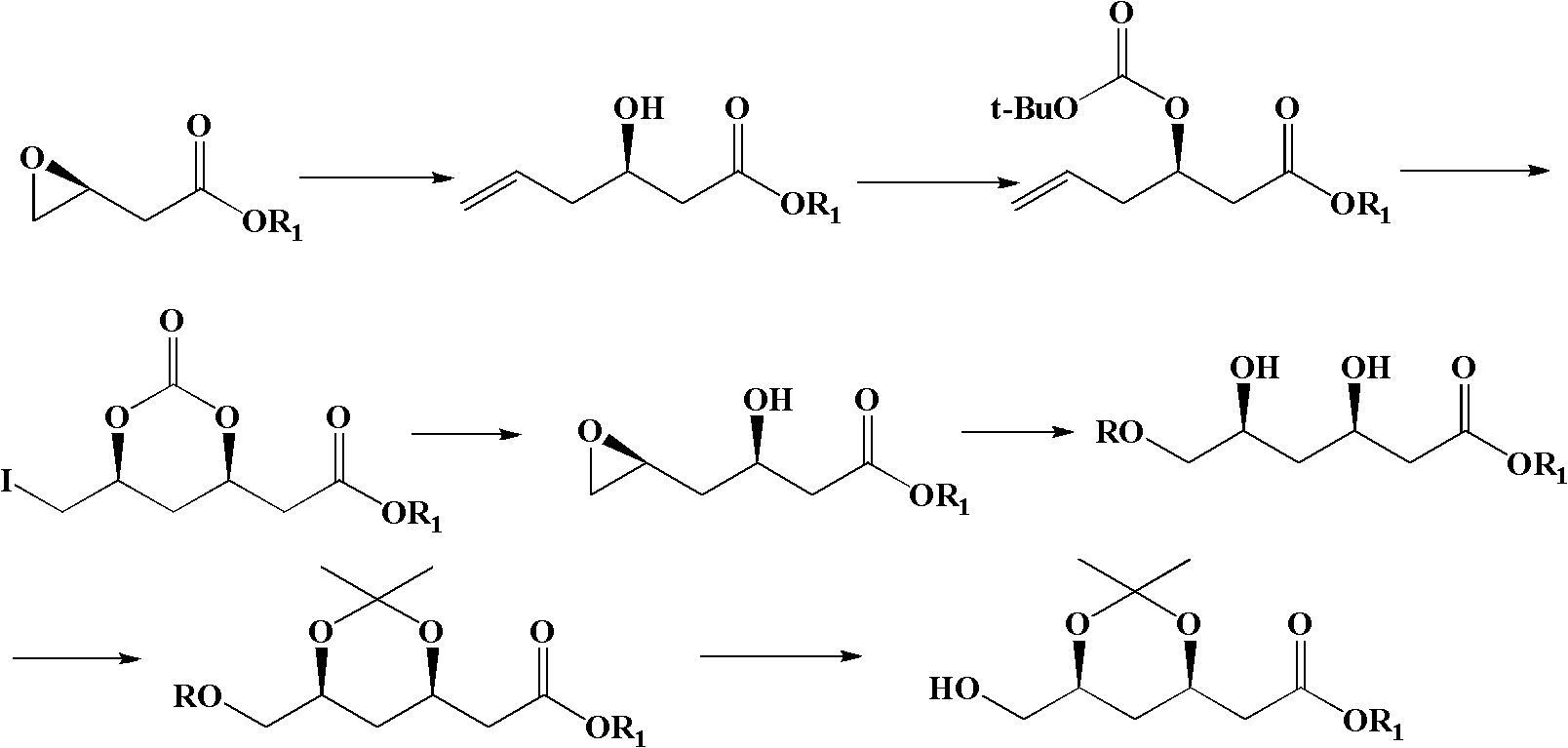
Method for preparing (4R-cis)-6-substituted-2,2-dimethyl-1,3-dioxane-4-tert-butyl acetate
A technology of tert-butyl acetate and 4r-cis, which is applied in the field of pharmaceuticals, can solve problems such as difficult control of the operation process and unsatisfactory de values of compounds, and achieve the effects of increasing yield and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The synthesis of embodiment 1 (S)-4-chloro-3-trimethylsilyloxybutyronitrile (compound II)
[0059] Under nitrogen protection, add 48.0g (0.4mol) (s)-4-chloro-3-hydroxybutyric acid ethyl ester, 59.2g (0.87mol) imidazole, 47.5g (0.44mol) trimethyl chloride Silane and 500 mL tetrahydrofuran were stirred at room temperature for 1 h, filtered with suction, and the filtrate was adjusted to neutrality with saturated ammonium chloride aqueous solution. Take the upper organic phase, wash the organic phase with water, and dry it. The solvent was evaporated to dryness to obtain 75.6 g, the yield was 98.6%, and the content measured by GC was 99.2%, which was (S)-4-chloro-3-trimethylsilyloxybutyronitrile (compound II).
Embodiment 2
[0060] The synthesis of embodiment 2 (S)-6-chloro-5-hydroxyl-3-oxohexanoic acid tert-butyl ester (compound III)
[0061] Under nitrogen protection, add 23.8g (0.36mol) of zinc powder, 0.3g (3mmol) of methanesulfonic acid, 150mL of tetrahydrofuran and 49.8g (0.26mol) of (S)-4-chloro-3 - Trimethylsiloxybutyronitrile (compound II) was heated to reflux for 10 min. Slowly add 60.8g (0.312mol) of tert-butyl bromoacetate dropwise within 1 hour, the reaction solution turns light yellow. After dropping, continue heating to reflux for 3h, cool to room temperature, and add 180mL of 3N hydrochloric acid dropwise. Stir at room temperature for 3 h, evaporate the solvent, and extract the residue with ethyl acetate, wash with water, and dry. The solvent was evaporated to dryness to obtain 54.6 g of a yellow oil, namely (S)-tert-butyl 6-chloro-5-hydroxy-3-oxohexanoate (compound III). The yield is 89.0%, and the content measured by GC is 98.6%.
Embodiment 3
[0062] The synthesis of embodiment 3 (R, S)-6-chloro-3,5-dihydroxyhexanoate (compound IV)
[0063] Under nitrogen protection, 51.9 g (0.22 mol) of (S)-6-chloro-5-hydroxy-3-oxohexanoic acid tert-butyl ester (compound III) obtained in the previous step, 450 mL of tetrahydrofuran, 360 mL methanol. Cool down to -78°C, add 44.6g of 50% methoxydiethylboron tetrahydrofuran solution dropwise, stir for 0.5h after dropping, add 9.7g (0.26mol) sodium borohydride in batches, and continue stirring at -78°C 6h, 50mL of glacial acetic acid was added dropwise. After dropping, stir and warm to room temperature. The reaction solution was poured into a mixture of 150mL ethyl acetate and 100mL water, the organic phase was taken, washed with water, dried, filtered with suction, and the solvent was evaporated to dryness to obtain 45.0g of a light yellow oil, which was (R,S)-6- Chloro-3,5-dihydroxyhexanoate (compound IV), yield 86.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com