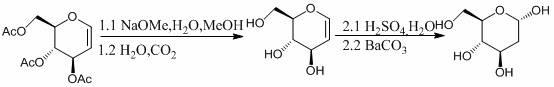
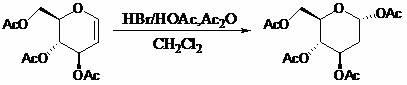Preparation method of 2-deoxidizing-D-glucose
A technology of glucose and glucone, applied in the field of preparation of 2-deoxy-D-glucose, can solve the problems of high cost, unsuitable for large-scale production and promotion, complicated steps and the like, and achieves low production cost, high yield, and high technology simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] a, the preparation of intermediates
[0028] Dissolve 1.08g of acetylglucosene in 5mL of dichloromethane, cool in an ice bath to 0°C, keep for 15 minutes, then remove the ice bath, and add hydrogen bromide / acetic acid (HBr / HOAc) with a concentration of 30% by mass in sequence Solution 0.2mL and acetic anhydride 10mL, stirred at 22°C for 24 hours for selective addition reaction, after the reaction was completed, add anhydrous sodium acetate to neutralize to pH = 7, stirred for 20 minutes, filtered and then evaporated under reduced pressure. The steaming temperature is 25°C and the pressure is 0.1MPa. The obtained solid is recrystallized with a solvent mixed with 10mL of petroleum ether and ethyl acetate at a volume ratio of 1:4, and then filtered and vacuum-dried under reduced pressure to obtain a white solid 2- Deoxy-1,3,4,6-tetra- O -Acetyl-D-glucose 1.30g, yield 99%.
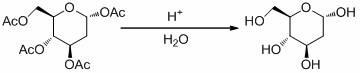
[0029] b. Synthesis of 2-deoxy-D-glucose
[0030] Take the intermediate 2-deoxy-1,3,4,6-tetra- O...
Embodiment 2
[0032] a, the preparation of intermediates
[0033] Dissolve 10.0 g of acetylglucosene in 50 mL of tetrahydrofuran, cool in an ice bath to 0°C, and keep for 50 minutes. Acetic anhydride 100mL, stirred at 30°C for 13 hours for selective addition reaction, after the reaction was completed, 50% (weight percent) sodium hydroxide was added to neutralize to pH = 7, stirred for 40 minutes, filtered and then rotary evaporated under reduced pressure, The rotary evaporation temperature is 25°C, the pressure is 0.1MPa, the obtained solid is recrystallized with 100mL of n-hexane and diethyl ether in a volume ratio of 1:4, and then filtered and vacuum-dried under reduced pressure to obtain a white solid 2-deoxy -1,3,4,6-four- O -Acetyl-D-glucose 10.9g, yield 90%.
[0034] b. Synthesis of 2-deoxy-D-glucose
[0035] Take the intermediate 2-deoxy-1,3,4,6-tetra- O Add 5.0 g of -acetyl-D-glucose to 20 mL of sulfuric acid with a concentration of 60% for acid-catalyzed hydrolysis reaction. Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com