Method for preparing vanadium pentoxide from sulfuric acid leach liquor of stone coal vanadium ore
A technology of vanadium pentoxide and leaching solution, applied in the direction of improving process efficiency, can solve the problems of ammonium salt or sodium salt pollution, excess impurities, ammonia pollution and other problems, and achieve the effect of ensuring purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
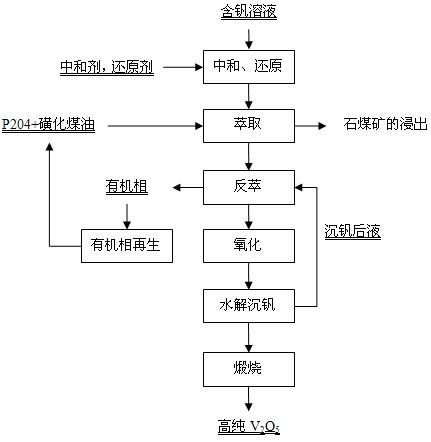
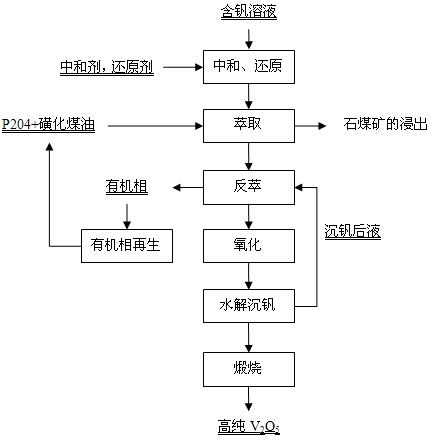
[0045] The leaching solution obtained by directly acid leaching stone coal vanadium ore with sulfuric acid contains 1.69g / L of vanadium. The vanadium solution obtained after neutralization and reduction pretreatment contains vanadium 1.60g / L, pH is 1.5, solution potential is 180mV, 10% P204+90% sulfonated kerosene (volume ratio) is used as the extraction organic phase, and the temperature is 35°C Compared with O / A=1:2.5 countercurrent 6-stage extraction, the raffinate contains less than 0.02g / L of vanadium. The loaded organic phase is back-extracted with 150g / L of sulfuric acid, and the back-extraction condition is compared to O / A=10:1, 4 stages of countercurrent back-extraction, and the stripping solution obtained contains vanadium 33.20g / L (fold V 2 o 5 59.23g / L), H 2 SO 4 80.38g / L. Add hydrogen peroxide to the stripping solution to oxidize to a solution potential of 900mV, maintain at 60°C for 30 minutes, then raise the temperature to 100°C, and continue to hydrolyze ...
Embodiment 2
[0047] The leaching solution obtained by direct acid leaching stone coal vanadium ore with sulfuric acid contains 6.2g / L of vanadium. The solution treated by Zhonghe reduction contains 6.1g / L of vanadium, pH is 2.3, and solution potential is 310mV. Using 25% P204+75% sulfonated kerosene (volume ratio) as the extraction organic phase, at a constant temperature of 35°C, compared O / A=1:1 countercurrent 6-stage extraction, the raffinate contains less than 0.05g / L of vanadium. The loaded organic phase was supplemented with acid to 150g / L after oxidative hydrolysis, and the stripping conditions were 8:1, 5-stage countercurrent stripping, and the obtained stripping solution contained vanadium 52g / L and H2SO4 40.29g / L. Hydrogen peroxide was added to the stripping solution, and it was oxidized and hydrolyzed at 100°C, and the vanadium precipitation was continuously hydrolyzed for 3 hours to obtain the vanadium precipitation intermediate product and the vanadium precipitation solution. ...
Embodiment 3
[0049] The leaching solution obtained by directly acid leaching stone coal vanadium ore with sulfuric acid contains 3.89g / L of vanadium. The vanadium solution obtained after neutralization and reduction pretreatment contains vanadium 3.60g / L, pH is 2.1, solution potential is 250mV, 15%P204+85%sulfonated kerosene (volume ratio) is used as the extraction organic phase, and the temperature is constant at 35°C Compared with O / A=1:1 countercurrent 6-stage extraction, the raffinate contains 0.02g / L of vanadium. The acidity of the loaded organic phase after oxidative hydrolysis is 137.03g / L, containing 8.54g / L of vanadium, and the stripping conditions are 10:1, 5-stage countercurrent stripping, and the obtained stripping solution contains 42.1g / L of vanadium, H2SO4 74.65g / L. Add persulfation to the stripping solution until the potential of the solution is 900mV, maintain it at 60°C for 30 minutes, then raise the temperature to 80°C, and continue to hydrolyze the vanadium precipitati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com