Method for organically catalyzing asymmetric conjugated addition of ketene and organic phosphorus compound
An organocatalytic enone and conjugate addition technology, applied in organic chemistry methods, asymmetric synthesis, organic compound/hydride/coordination complex catalysts, etc., can solve problems such as space congestion, poor selectivity, and difficult problems. Achieve high catalytic efficiency, less catalyst consumption and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
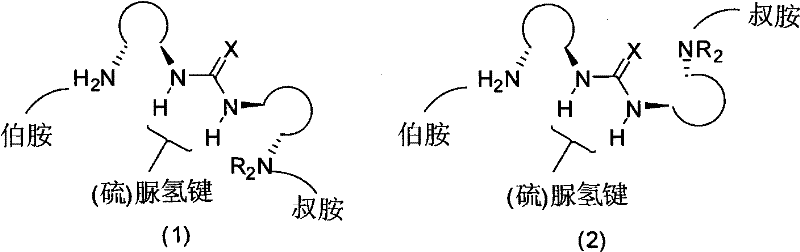
[0038] In 2 mL of dichloromethane, add cyclohexenone (0.288 g, 3.0 mmol), diphenoxyphosphine (0.202 g, 1 mmol), 1,2-diaminocyclohexane (R, R) and quinine to construct Primary thiourea catalyst (R 2 is quinine, X is sulfur, cyclohexanediamine is R, R configuration) (0.048g, 0.1mmol), after the addition of the material, the reaction conversion is complete after stirring at 20°C to 25°C for 48h. The reaction solution was concentrated under reduced pressure and separated by silica gel column chromatography (petroleum ether / ethyl acetate=3:1) to obtain 0.280 g of a white solid with a yield of 94% and ee=90%. 1 HNMR (400MHz, CDCl 3 ): δ (ppm) 7.78-7.73 (m, 4H), 7.52-7.50 (m, 6H), 2.81-2.62 (m, 2H), 2.39-2.34 (m, 2H), 2.30-2.28 (m, 1H) , 2.21-2.17(m, 1H), 1.96-1.83(m, 2H), 1.72-1.69(m, 1H). 13 CNMR (100MHz, CDCl 3): δ (ppm) 209.8, 209.6; 131.9; 131.9; 130.9; 130.8; 128.9; 128.8; 41.2; 39.3; 38.1; 26.4; 23.3. HRMS (ESI): Theoretical M + (C 18 h 19 o 2 P+H) was m / z 299.1201, a...
Embodiment 2
[0040] The difference from Example 1 is that cyclohexenone (0.0288g, 0.3mmol), diphenoxyphosphine (0.020g, 0.1mmol), 1,2-diaminocyclohexane (R, R) and quinine The constructed primary thiourea catalyst (R 2 is quinine, X is sulfur, cyclohexanediamine is R, R configuration) (0.0048g, 0.01mmol), the yield is 93%, ee=90%.
[0041] Implementation example 2
[0042] The difference from Example 1 is that cyclohexenone (0.0288g, 0.3mmol), diphenoxyphosphine (0.020g, 0.1mmol), 1,2-diaminocyclohexane (R, R) and quinine The constructed primary thiourea catalyst (R 2 is quinine, X is sulfur, cyclohexanediamine is R, R configuration) (0.0048g, 0.01mmol), the yield is 93%, ee=90%.
Embodiment 3
[0044] The difference from Example 1 is that cyclohexenone (0.192g, 2.0mmol), diphenoxyphosphine (0.202g, 1mmol), 1,-diaminocyclohexane (R, R) and quinine are added to construct The primary thiourea catalyst (R 2 is quinine, X is sulfur, cyclohexanediamine is R, R configuration) (0.048g, 0.1mmol), the yield is 93%, ee=90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com