Oxide catalyst for producing Alpha-Alpha-dimethylphenyl carbinol and preparation method thereof
A technology of dimethylbenzyl alcohol and oxide, applied in the field of oxide catalyst and preparation thereof, can solve problems such as affecting product quality, reducing yield, affecting separation process, etc., and achieves avoiding thermal decomposition, proper distribution of active sites and reaction low temperature effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Weigh 126.4g KMnO respectively at room temperature 4 , 20.2g Fe(NO 3 ) 3 9H 2 O, 2.41gCu(NO 3 ) 2 , 8.76gCo(C 2 o 4 ) 2 2H 2 O, and the above compound was dissolved in 1.0L deionized water, then concentrated nitric acid was added to the above solution to adjust the pH to about 1.0, and 22.3g of Mn(NO 3 ) 2 and added to the above solution, and stirred, refluxed for 6 hours; then with concentrated HNO 3 Adjust the pH value of the reaction system to 8.0 with NaOH, transfer the resulting mixture to an autoclave, keep the temperature at 150°C for 48 hours, filter, wash and dry the product obtained after constant temperature, and then dry the dried product at 1mol / Soak in L of nitric acid for 1-8 hours, then filter and dry again; and bake at 400° C. for 4-24 hours in a nitrogen atmosphere to obtain the catalyst precursor I. The catalyst precursor I was mixed with alumina powder at a weight ratio of 10:1, an appropriate amount of nitric acid was added, and extruded...
Embodiment 2~10
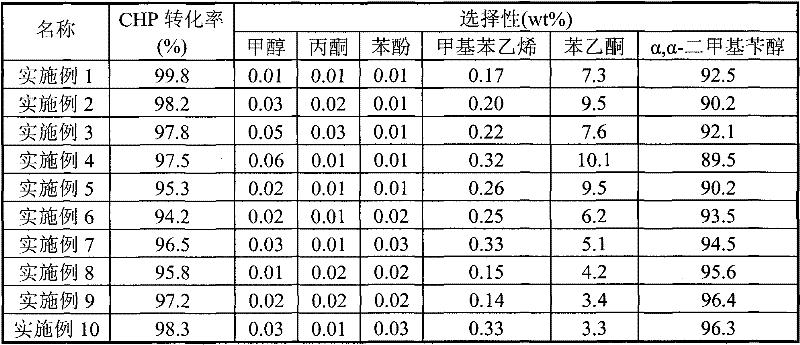
[0023] Prepare the catalyst and evaluate the catalyst according to the various steps and conditions obtained in Example 1, but only change the synthesis conditions such as the type of raw materials, the ratio of raw materials, synthesis conditions, roasting conditions and catalyst molding factors, and list the synthesis conditions in Table 1; The catalysts obtained by different synthesis methods were evaluated under the same evaluation conditions, and the evaluation results are listed in Table 2.
[0024] Table 1
[0025]
name
Raw material type
Catalyst composition
resolve resolution
+Roasting conditions+Molding
Example 1
KMnO 4 +Fe(NO 3 ) 3 +Co(NO 3 ) 2
+Cu(NO 3 ) 2 +Mn(NO 3 ) 2
80% Manganese Oxide, 10% Iron Oxide,
0.1% copper oxide, 0.1% cobalt oxide,
0.8% sodium oxide, 9% aluminum oxide
pH=3.0,
Air+180℃+alumina
Example 2
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com