Method for producing Alpha, Alpha-dimethyl phenyl carbinol
A technology of dimethyl benzyl alcohol and cumene hydroperoxide, applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., can solve problems such as pollution, sulfur-containing environment of products, and reduce decomposition temperature , high catalytic activity, good redox effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] Weigh 126.4g KMnO respectively at room temperature 4 , 20.2g Fe(NO 3 ) 3 9H 2 O, 2.41gCu(NO 3 ) 2 , 8.76gCo(C 2 o 4 ) 2 2H 2 O, and above-mentioned compound is dissolved in 1.0L deionized water, then in above-mentioned solution, add concentrated nitric acid, adjust pH to about 1.0, weigh the MnSO of 22.3g simultaneously 4 4H 2 O and added to the above solution, and stirred, refluxed for 6 hours; then with concentrated HNO 3 Adjust the pH value of the reaction system to 8.0 with NaOH, transfer the resulting mixture to an autoclave, keep the temperature at 150°C for 48 hours, filter, wash and dry the product obtained after constant temperature, and then dry the dried product at 1mol / Soak in L of nitric acid for 1-8 hours, then filter and dry again; and bake at 400° C. for 4-24 hours in a nitrogen atmosphere to obtain the catalyst precursor I. Mix catalyst precursor I and alumina powder according to the weight ratio of 4:1, add an appropriate amount of nitric a...
Embodiment 2~10
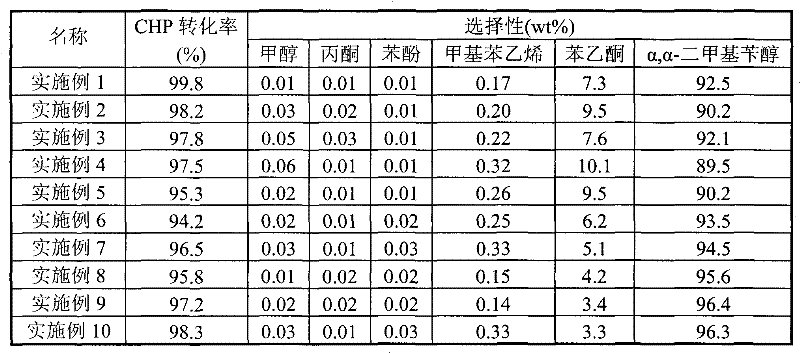
[0013] Prepare the catalyst and evaluate the catalyst according to the various steps and conditions obtained in Example 1, but only change the synthesis conditions such as the type of raw materials, the ratio of raw materials, synthesis conditions, roasting conditions and catalyst molding factors, and list the synthesis conditions in Table 1; The catalysts obtained by different synthesis methods were evaluated under the same evaluation conditions, and the evaluation results are listed in Table 2.
Embodiment 11~20
[0015] Prepare the catalyst and evaluate the catalyst according to the various steps and conditions of Example 1, but change the evaluation parameters such as the concentration of cumene hydroperoxide, reaction temperature, system pressure, liquid phase space velocity and solvent, and list the evaluation results in the table 3 in.
[0016] Table 1
[0017] name
Raw material type
Catalyst composition
Synthesis method + roasting conditions + molding
Example 1
KMnO 4 +Fe(NO 3 ) 3 +Co(NO 3 ) 2 +Cu(NO 3 ) 2 +Mn(NO 3 ) 2
80% manganese oxide, 10% iron oxide, 0.1% copper oxide, 0.1% cobalt oxide, 0.8% sodium oxide, 9% aluminum oxide
pH=3.0, air+180℃+alumina
Example 2
K 2 MnO 4 +Fe(NO 3 ) 3 +Co(C 2 o 4 ) 2 +Cu(NO 3 ) 2 +Mn(NO 3 ) 2
80% manganese oxide, 1% iron oxide, 1% copper oxide, 1% cobalt oxide, 2% sodium oxide, 15% aluminum oxide
pH=1.0, air+240℃+aluminum sol
Example 3
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com