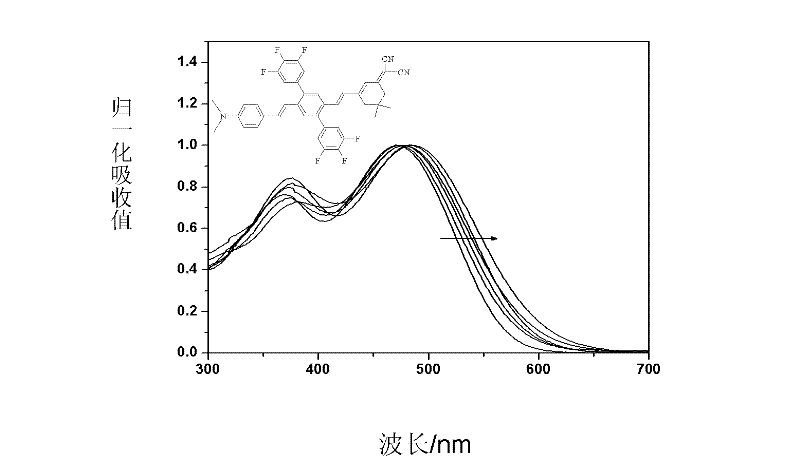
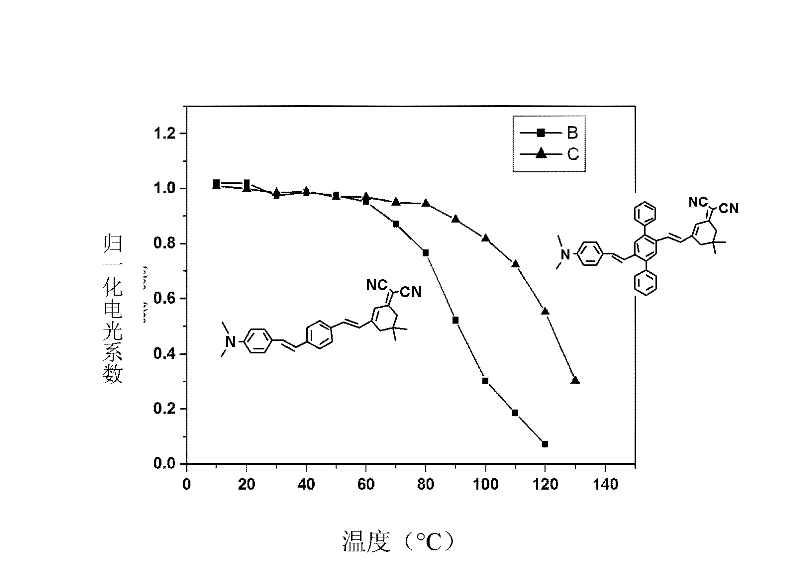
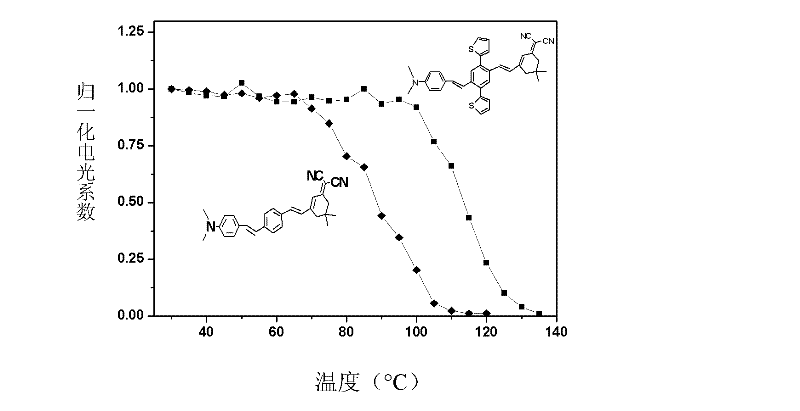
Two-dimensional spindly non-linear optic chromophore molecule
A nonlinear optical and spindle-shaped technology, applied in organic chemistry, luminescent materials, chemical instruments and methods, etc., can solve the problems of low phase matching ability, small rotation radius, etc., and achieve the effect of improving the stability of nonlinear optics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The synthetic route of the two-dimensional spindle-shaped chromophore molecule is as follows:
[0059]
[0060] Synthesis of [1,1'; 4',1"]terphenyl-2',5'-dialdehyde
[0061] The compound triphenylphosphine (0.015g, 0.06mmol), palladium acetate (0.002g, 0.01mol), phenylboronic acid (0.24g, 2mmol), 2,5-dibromo-1,4-terephthalaldehyde (0.29 g, 1mmol) of the mixture was added to a 100ml three-necked flask, and nitrogen protection was introduced. Then isopropanol (10ml), 2N / L sodium carbonate solution (3ml) and water (1ml) were transferred to the flask, stirred, and the reaction system was heated to 70°C for 4 hours. After extraction with dichloromethane, wash once with saturated sodium bicarbonate solution, concentrate and dry under vacuum to obtain a light yellow solid. Purified by column chromatography (eluent: ethyl acetate: petroleum ether = 1:30) to obtain 0.25 g of white crystals with a yield of 95%. 1 HNMR (500MHz, CDCl 3 , TMS): δ (ppm) 7.44-7.46 (d, 4H, J ~ 9...
Embodiment 2
[0068]
[0069] Synthesis of 2,5-(3,4,5-trifluorophenyl)-1,4-dicarbaldehyde:
[0070] 2,5-dibromobenzene-1,4-dicarbaldehyde (0.5g, 1.72mmol) and 3,4,5-trifluorophenylboronic acid (0.596g, 4.11mmol) were dissolved in tetrahydrofuran (15mL), and nitrogen gas Protect. Add palladium acetate (6mg, 0.026mmol) to the solution, triphenylphosphine (20.52mg, 0.078mmol), 2M sodium carbonate (15.3mL, 40mmol), deionized water (14mL), heat to reflux, and React for 6 hours. The layer was concentrated under reduced pressure and purified by column chromatography with petroleum ether-ethyl acetate (20:1) as the eluent to obtain 0.4 g (60%) of a yellow solid.
[0071] Synthesis of 2,5-(3,4,5-trifluorophenyl)-4-(4-(dimethylamino)styryl)benzaldehyde:
[0072] 2,5-(3,4,5-trifluorophenyl)-1,4-dicarbaldehyde (0.4g, 1.02mmol) was dissolved in 20mL of dichloromethane, and then iodide (4-dimethyl Amino-benzylidene)-triphenylphosphonium salt (0.532g, 1.02mmol) and potassium tert-butoxide (0.172g, ...
Embodiment 3
[0077] Synthesis of [1,1'; 4',1"]terphenyl-2',5'-dialdehyde
[0078] Same as Example 1
[0079] Synthesis of 2-[4-(N,N-dimethylamino)styryl]-1,4-diphenylbenzaldehyde
[0080] Same as Example 1
[0081] Synthesis of 2-[4-(N,N-dimethylamino)styryl]-1,4-diphenylstyryltricyanoethenyl-2,2-dimethyldihydrofuran
[0082] Dissolve 2-[4-(N,N-dimethylamino)styryl]-1,4-diphenylbenzaldehyde (0.52 g, 1 mmol) in dry THF (3 ml), then add tricyano Vinyl-1-methyldihydrofuran (0.2g, 1.0mmol) and piperidine (0.03ml), the reaction system was stirred at room temperature for 2 hours, and the temperature was raised to 90°C to continue the reaction for 2 hours. A purple solution was obtained. The progress of the reaction was monitored by TLC. After the reaction was completed, it was washed with water, extracted three times with dichloromethane, and a red solid was obtained after the solvent was concentrated. The crude product was purified by column chromatography (petroleum ether: ethyl acetate =...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com