Vincristine nano targeting slow-release long-circulation liposome and preparation method thereof
A long-circulation liposome and vincristine technology, applied in the field of medicine, can solve the problems of easy fusion and aggregation during storage and application, difficulty in multi-functional modification, poor physical and chemical stability, etc., to achieve adjustable sustained release time, The effect of short preparation cycle and strong drug loading capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
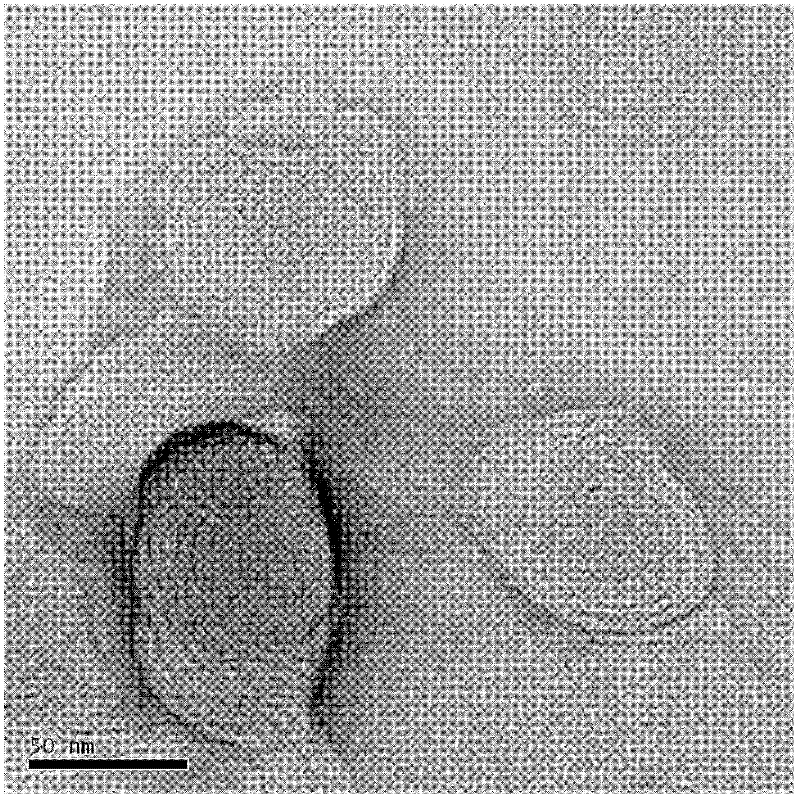
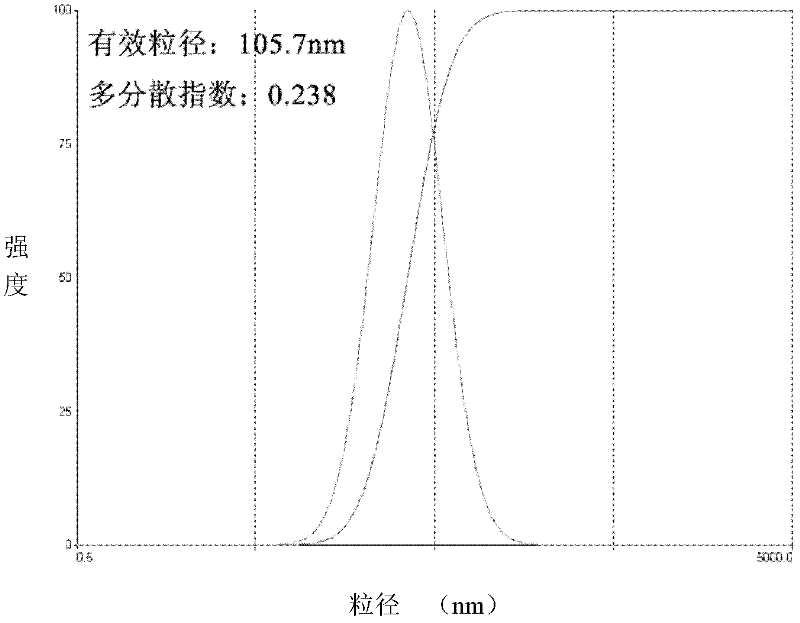
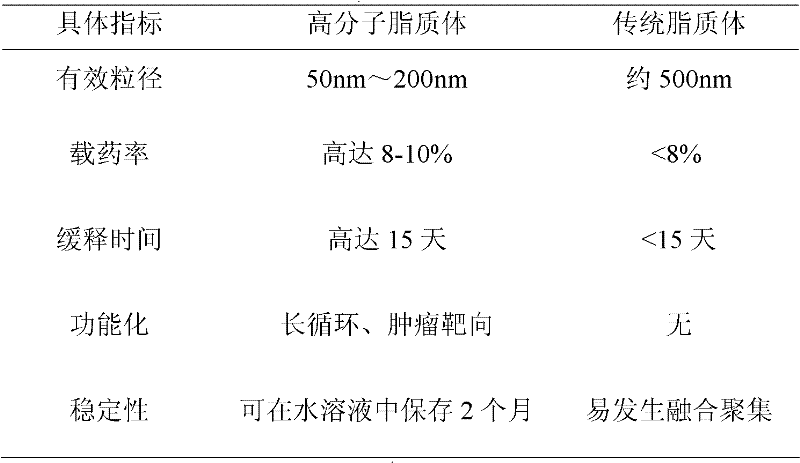
Image
Examples
Embodiment 1
[0031] PEG-OQLCS preparation process. Weigh 0.3g of OQLCS and dissolve in 10mL of distilled water for use; weigh 0.2g of monocarboxylated polyethylene glycol and dissolve in 10mL of distilled water, weigh 1g of EDC and add it to the aqueous solution of monocarboxylated polyethylene glycol, then weigh NHS 0.6g was added to monocarboxylated polyethylene glycol aqueous solution, and after 10 minutes, the above solution was added to OQLCS aqueous solution, and reacted for 12 hours. After the reaction, dialyzed with 8000-14000 dialysis bag for 1 day, and freeze-dried to obtain PEG- OQLCS.
[0032] FA-OQLCS preparation process. Folic acid was dissolved in 60 mL of DMSO, 1 mL of triethylamine was added thereto, 1 g of EDC and 0.6 g of NHS were dissolved with a small amount of DMSO, and then added to the above reaction system for 12 h in the dark. The product was filtered to obtain NHS-FA active lipid as a yellow solid. Dissolve NHS-FA active lipid in 50mL DMSO, and add 0.3g OQLCS....
Embodiment 2
[0034]PEG-OQLCS preparation process. Weigh 0.4g of OQLCS and dissolve in 10mL of distilled water for use; weigh 0.6g of monocarboxylated polyethylene glycol and dissolve in 10mL of distilled water, weigh 2g of EDC and add it to the aqueous solution of monocarboxylated polyethylene glycol, then weigh NHS 1.3g was added to monocarboxylated polyethylene glycol aqueous solution, and after 25 minutes, the above solution was added to OQLCS aqueous solution, and reacted for 18 hours. After the reaction, dialyzed with 8000-14000 dialysis bag for 4 days, and freeze-dried to obtain PEG- OQLCS.
[0035] FA-OQLCS preparation process. Dissolve folic acid in 80 mL of DMSO, add 3 mL of triethylamine to it, dissolve 2 g of EDC and 1.3 g of NHS with a small amount of DMSO, and then add them to the above reaction system to avoid light for more than 18 h. The product was filtered to obtain NHS-FA active lipid as a yellow solid. Dissolve NHS-FA active lipid in 65mL DMSO, and add 0.4g OQLCS. u...
Embodiment 3
[0037] PEG-OQLCS preparation process. Weigh 0.5g of OQLCS and dissolve it in 10mL distilled water for use; weigh 1.0g of monocarboxylated polyethylene glycol and dissolve it in 10mL of distilled water, weigh 3g of EDC and add it to the aqueous solution of monocarboxylated polyethylene glycol, then weigh NHS 2.0g was added to the monocarboxylated polyethylene glycol aqueous solution, and after 40 minutes, the above solution was added to the OQLCS aqueous solution, and reacted for 24 hours. After the reaction, dialyzed with 8000-14000 dialysis bag for 7 days, and freeze-dried to obtain PEG- OQLCS.
[0038] FA-OQLCS preparation process. Dissolve folic acid in 100mL of DMSO, add 5mL of triethylamine to it, dissolve 3g of EDC and 2.0g of NHS with a small amount of DMSO, and add them to the above reaction system to avoid light for more than 24h. The product was filtered to obtain NHS-FA active lipid as a yellow solid. Dissolve NHS-FA active lipid in 80mL DMSO, and add 0.5g OQLCS....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Effective particle size | aaaaa | aaaaa |
| Effective particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com