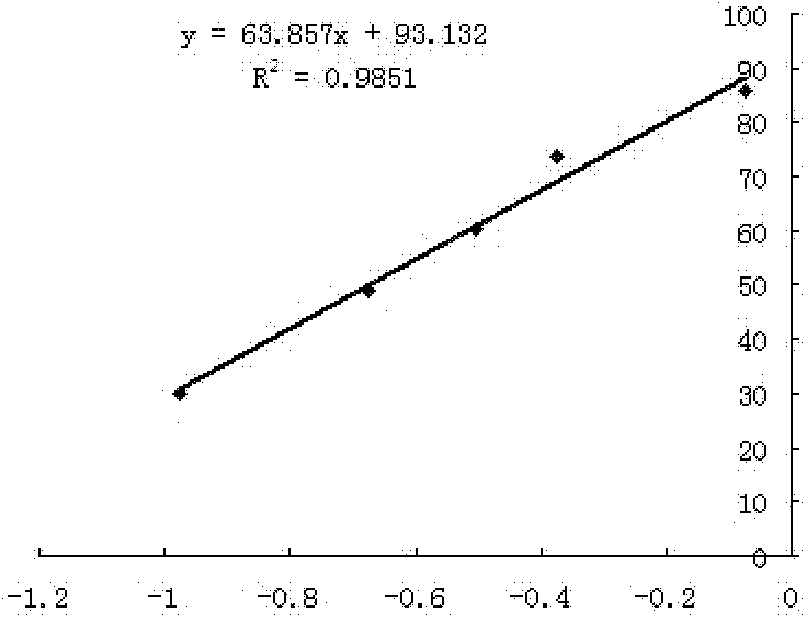
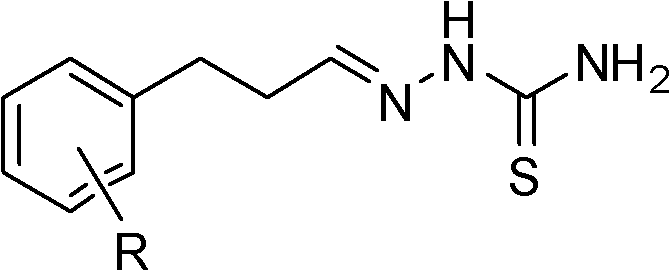
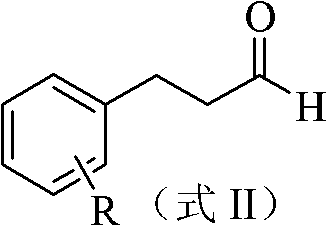
3-substituted phenyl propionaldehyde thiosemicarbazone compounds, and preparation method and application thereof
A compound and 3-br technology, applied in the field of 3-substituted phenylpropionaldehyde thiosemicarbazide compounds, can solve the problems of unstable chemical properties of L-ascorbic acid, obvious side effects, poor inhibitory effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Preparation of 3-(2,4-dichlorophenyl)-propionaldehyde thiosemicarbazone (S102)
[0024] In the 50mL there-necked flask, add 0.22g (1.09mmol) compound shown in formula II (R is 2, the dichloro that 4 positions replace) 3-(2,4-dichlorophenyl)-propionaldehyde, 20mL organic solvent ethanol and 0.11g (1.2mmol) of thiosemicarbazide, 0.6mg (0.01mmol) of acidic compound acetic acid was added at one time, heated to 78°C under reflux for condensation reaction for 5 hours, and then stopped the reaction. The reaction solution was spin-dried and recrystallized from ethanol to obtain 0.2 g of white granular solid, yield: 66%. The appearance and fusing point of this white granular solid product are shown in Table 1, and its 1 H NMR spectrum data are shown in Table 2. It can be seen from Table 2 that the product has a correct structure and is a compound shown in Formula I, numbered S102.
[0025]
[0026] (Formula I)
[0027] Among them, R is 2,4-Cl 2 .
[0028] Accor...
Embodiment 2
[0029] Example 2: Preparation of 3-(4-chlorophenyl)-propionaldehyde thiosemicarbazone (S103)
[0030] Add 0.42g (2.50mmol) compound shown in formula II (R is 4-substituted chlorine) 3-(4-chlorophenyl)-propionaldehyde in 50mL three-necked flask, 10mL organic solvent ethanol and 0.22g (2.42mmol) amino Thiourea, add 6 mg (0.1 mmol) of acetic acid compound, heat to 78 ° C under reflux for condensation reaction for 2 hours, stop the reaction. The reaction solution was spin-dried, and recrystallized from ethanol to obtain 0.49 g of white crystals, with a yield of 82%. The appearance and fusing point of this white crystal product are shown in Table 1, and its 1 H NMR spectrum data are shown in Table 2. It can be seen from Table 2 that the product has a correct structure and is a compound shown in Formula I, numbered S103.
[0031]
[0032] (Formula I)
[0033] Wherein, R is 4-Cl.
[0034] According to the same method as above for the preparation of compound S103, only the com...
Embodiment 3
[0041] Example 3: 3-substituted phenylpropionaldehyde thiosemicarbazones inhibit activity of cotton bollworm tyrosinase
[0042] Enzyme solution preparation: add 1 head of 5th instar cotton bollworm larvae to a glass homogenizer pre-cooled in ice-water mixture, 1ml 0.1mol / l, phosphate buffer saline solution with pH value of 6.5, fully homogenate, and place in a centrifuge Centrifuge at a speed of 10800 rpm for 15 minutes, and after suction filtration, take the supernatant in an ice bath for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com