Improved method for preparing entecavir
A technology of entecavir and compounds, applied in the field of improved preparation of antiviral drug entecavir, can solve the problems of excessive waste water and waste solvent, low total yield, unfavorable environmental protection, etc., and achieve high application value, good reproducibility, and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 (2R, 3S, 5S)-3-benzyloxy-5-[6-benzyloxy-2-[[[(4-methoxyphenyl) diphenylmethyl]amino]-9H Preparation of -purin-9-yl]-2-[(benzyloxy)methyl]-cyclopentanone
[0026]
[0027] Among them, MMT represents 4-methoxytrityl, Bn represents benzyl
[0028] Add (1S, 2S, 3S, 5S)-3-benzyloxy-5-[-6-benzyloxy-2-[[(4-methoxyphenyl)benzhydryl] into a 1000ml three-necked flask -Amino]-9H-purin-9-yl]-2-[(benzyloxy)methyl]-cyclopentanol (40g, 48.6mmol) and dichloromethane (600ml), stirred and dissolved, and saturated carbonic acid was added under stirring Aqueous sodium hydrogen solution (120ml), stirred for 10 minutes. Then iodine (26 g, 102.4 mmol) and TEMPO (2,2,6,6-tetramethylpiperidin-1-oxyl radical) (1.0 g, 6.4 mmol) were added, and the reaction solution was stirred at 20° C. for 5 hours. Cool to 0°C, add 10% sodium sulfite solution (200ml), and stir for 15 minutes. The layers were separated, the aqueous layer was extracted with ethyl acetate (160ml), the organic phase...
Embodiment 2
[0029] Example 2 (1S, 3R, 4S)-6-benzyloxy-9-[[2-methylene-4-benzyloxy-3-(benzyloxy)methyl]-cyclopentyl]-N - Preparation of [(4-methoxyphenyl)benzhydryl]-9H-purin-2-amine (formula II, R=4-methoxytrityl)
[0030]
[0031] Formula II
[0032] Wherein, MMT represents 4-methoxytrityl, and Bn represents benzyl.
[0033] Under nitrogen atmosphere, magnesium (8.14g, 334.8mmol) and dichloromethane (184ml, 2872mmmol) were added to the three-necked flask, cooled to 0°C, and titanium tetrachloride (18.4ml, 167.4mmol) was added with stirring. (2R, 3S, 5S)-3-benzyloxy-5-[6-benzyloxy-2-[[[(4-methoxyphenyl)diphenylmethyl]amino]-9H-purine -9-yl]-2-[(benzyloxy)methyl]-cyclopentanone (prepared in Example 1) (38.4g, 46.8mmol) in dichloromethane (184ml, 2872mmol) and tetrahydrofuran (154ml, 1900mmol) ) was added dropwise into the reaction solution, the rate of addition was controlled so that the internal temperature was not higher than 5°C, and the mixture was stirred at 0°C for 20 minutes a...
Embodiment 3
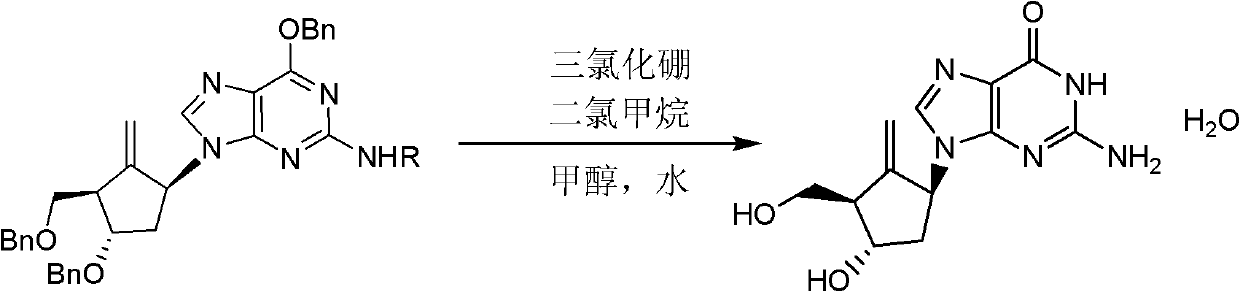
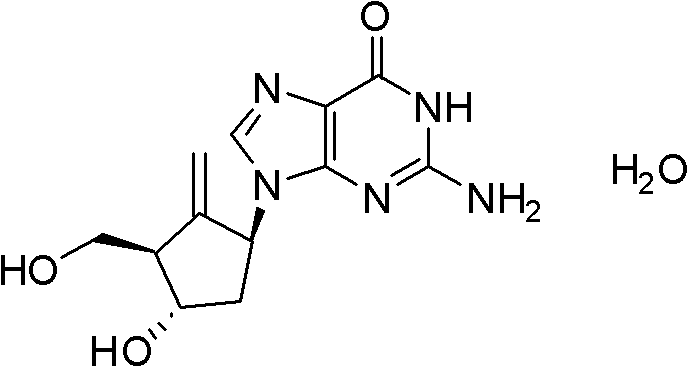
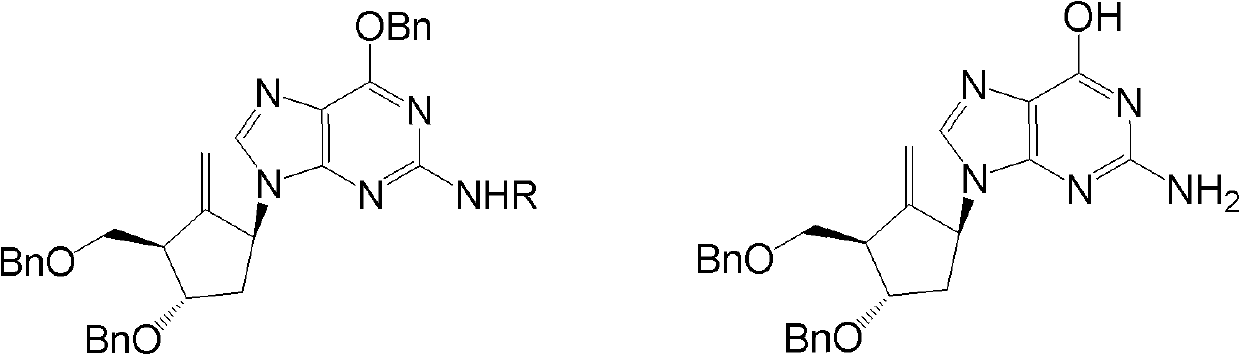
[0035] Example 3 Preparation of Entecavir
[0036]
[0037] Formula II (R=4-methoxytrityl) Formula I
[0038] Add the compound of formula II (R=4-methoxytrityl)(1S,3R,4S)-N-[(4-methoxyphenyl)benzhydryl to a 1L three-necked flask under nitrogen protection ]-6-benzyloxy-9-[2-methylene-4-benzyloxy-3-(benzyloxy)methyl]cyclopentyl]-9H-purin-2-amine (from Example 2 Prepared) (15.58g, 0.019mol) and dichloromethane (450ml), stirred and dissolved, cooled the reaction solution to -75~-80°C, added dropwise 1mol / l boron trichloride dichloromethane solution (228ml, 0.228 mol), gradually warm up to -25~-30°C and stir for 1 hour after dropping.
[0039]The reaction solution was cooled to -60~-65°C, methanol (300ml, 7.406mol) and water (30.8ml, 1.710mol) were added dropwise, the temperature was gradually raised while stirring, and dichloromethane was distilled off under reduced pressure. The temperature of the reaction solution was raised to 55-60°C and stirred for 1 hour. After distil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com