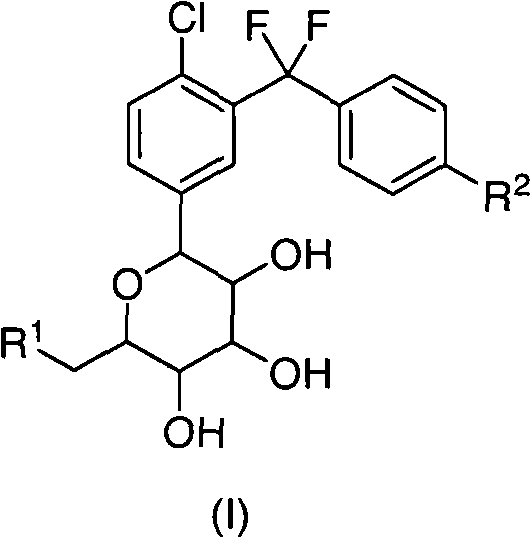
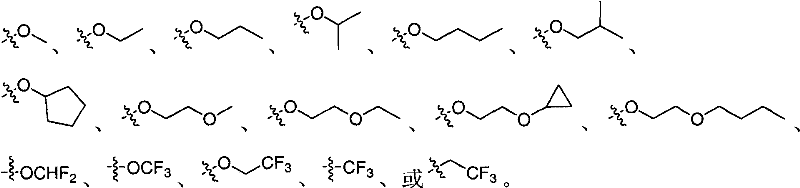
C-aryl glucoside derivatives containing difluoromethylene group
A difluoromethoxy and trifluoromethyl technology, applied in the field of diabetes treatment, can solve the problems of unsatisfactory target enzyme selectivity, inactivation, and unsatisfactory SLGT-2 inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] In the preparation method of the present invention, each reaction is usually carried out in an inert solvent (usually a polar aprotic solvent) at -30°C to solvent reflux temperature (preferably -20 to 80°C). The reaction time is usually 0.1 to 60 hours, preferably 0.5 to 48 hours.
[0063] In a preferred example, the compound of formula (I) of the present invention can be prepared according to the following routes I-VII.
[0064] Route I: Synthesis of Intermediate 1i
[0065]
[0066] (1) At a suitable temperature, in a polar aprotic solvent, add a Lewis acid as a catalyst, and react 5-bromo-2-chlorobenzoyl chloride with tert-butyl (phenoxy)diphenylsilane to obtain an intermediate 1a. Polar aprotic solvents may be selected from (but not limited to): dichloromethane, chloroform, 1,2-dichloroethane; Lewis acids may be selected from (but not limited to): aluminum trichloride, dichloro Zinc chloride, ferric chloride, tin tetrachloride, titanium tetrachloride; the pref...
specific Embodiment approach
[0128] The present invention is explained more specifically in the following examples. It should be understood, however, that these examples are given to illustrate the invention and not to limit the scope of the invention in any way. For the experimental methods without specific conditions indicated in the following examples, the conventional conditions or the conditions suggested by the manufacturer are usually followed. Parts and percentages are by weight unless otherwise indicated.
[0129] In all examples, the melting point is determined with an X-4 melting point apparatus, and the thermometer is not corrected; 1 H-NMR was recorded with a VarianMercury 400 nuclear magnetic resonance instrument, and the chemical shift was expressed in δ (ppm); MS was measured with a Shimadzu LC-MS-2020 mass spectrometer. The silica gel used for separation is not specified and is 200-300 mesh, and the ratio of the eluent is the volume ratio.
Embodiment 1
[0131] (2S, 3R, 4R, 5S, 6R)-2-(4-chloro-3-((4-ethoxyphenyl)difluoromethyl)phenyl)-tetrahydro-6-(hydroxymethyl) - Preparation of 2H-pyran-3,4,5-triol (compound 1)
[0132] Step 1: Preparation of (5-bromo-2-chlorophenyl)(4-(tert-butyl(phenoxy)diphenylsilane)phenyl)methanone 1a
[0133]
[0134] Dissolve 5-bromo-2-chlorobenzoyl chloride (12.7g, 50mmol) in dichloromethane (100mL), and add tert-butylphenoxydiphenylsilane (16.6g, 50mmol) and trichloromethane in sequence at 0°C Aluminum (8.0g, 60mmol), stirred at room temperature for 3.5h, TLC monitored the completion of the reaction, washed with 1N hydrochloric acid, 1N sodium hydroxide, water, saturated sodium chloride, and dried over anhydrous sodium sulfate. After suction filtration, the solvent was evaporated under reduced pressure, and purified by column chromatography to obtain the title compound 1a as a white solid (10.1 g, 36.7%). 1 H-NMR (300MHz, CDCl 3 ): δ1.11(s, 9H), 6.79-6.82(m, 2H), 7.26-7.51(m, 9H), 7.57-7.60(m,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com