Human epidermal growth factor nanoliposome and its preparation method
A technology of epidermal growth factor and nano-liposome, which is applied in skin care preparations, skin diseases, pharmaceutical formulations, etc., can solve the problems of unstable and long-acting release, hEGF cannot be quickly and completely absorbed by tissues, and inactivated. Achieve good epidermal penetration, improve transdermal absorption, and improve stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] According to the prescription volume 1ml unit, the prescription is as follows:
[0067] Prescription one:
[0068] Human Epidermal Growth Factor 1mg
[0069] Lecithin 182mg
[0070] Cholesterol (pharmaceutical grade) 18mg
[0071] Chloroform (analytical grade) 5ml
[0072] Phosphate buffer 1ml
[0073] Accurately weigh 182mg of lecithin, 18mg of cholesterol (PC: CHOL = 10: 1) dissolved in 10ml of chloroform ultrasonic (time 5min, frequency 53kHz) dissolved, after thorough mixing, add 1ml of hEGF aqueous solution (1mg / ml, drug substance ratio = 1: 100), ultrasonically mixed for about 4 minutes to form a W / O emulsion, decompressed (vacuum degree 800mbar, temperature 40°C) to remove the organic solvent to form a colloidal substance, then added 1ml of phosphate buffer, and ultrasonicated for 10min to obtain 1ml milky white uniform hEGF nano liposomes.
[0074] Preparation sample appearance: milky white homogeneous liquid
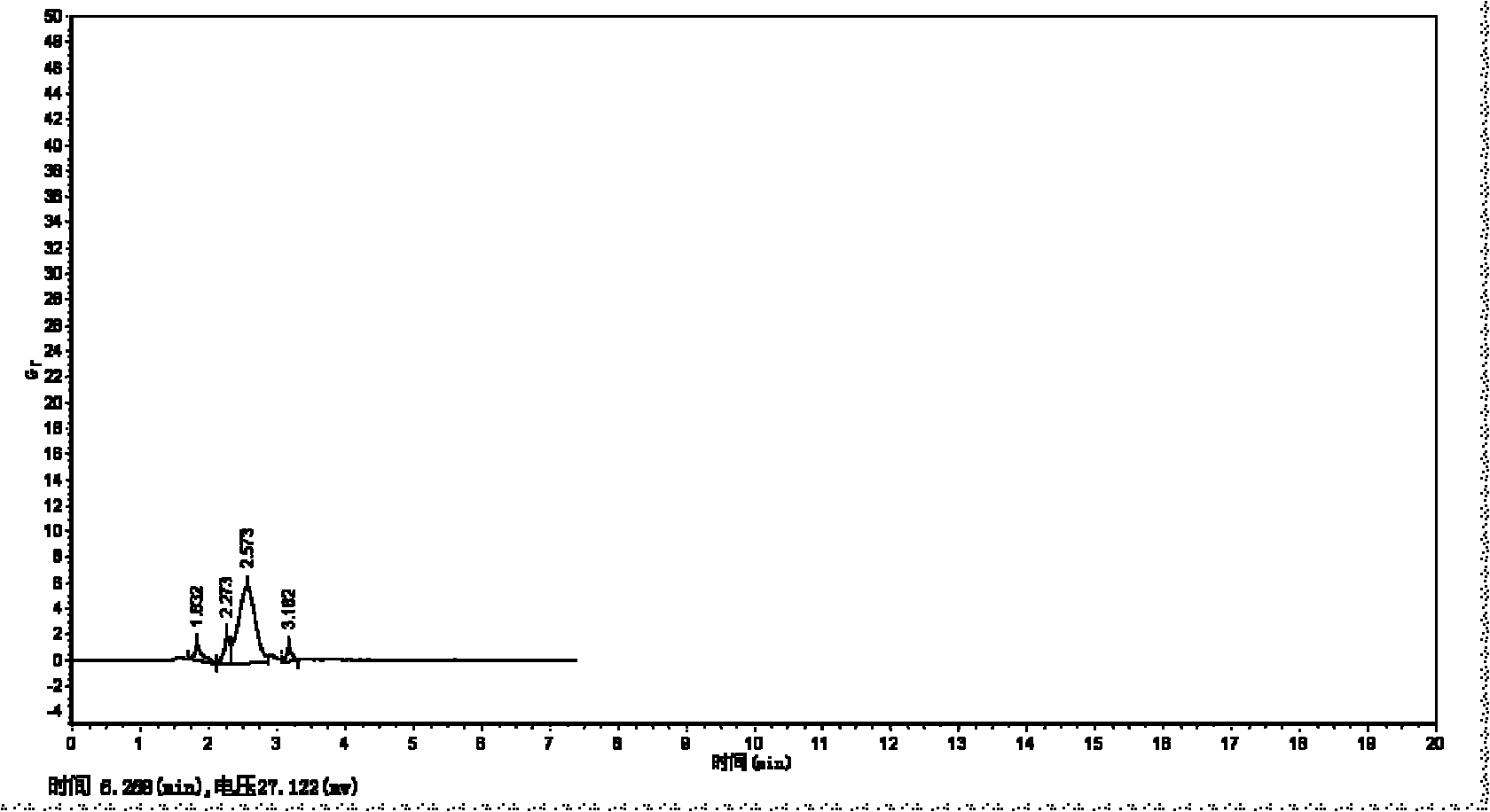
[0075] Encapsulation efficiency result: 30.8...
Embodiment 2
[0080] Prescription two:
[0081] According to the prescription volume 1ml unit, the prescription is as follows:
[0082] Human Epidermal Growth Factor 1mg
[0083] Lecithin 80mg
[0084] Cholesterol (pharmaceutical grade) 20mg
[0085] Chloroform (analytical grade) 5ml
[0086] Phosphate buffer 1ml
[0087] Preparation process parameters:
[0088] Accurately weigh 80mg of lecithin, 20mg of cholesterol (PC: CHOL = 4: 1) and dissolve in 5ml of chloroform by ultrasonic (time 2min, frequency 35kHz) to dissolve, after mixing thoroughly, add 1ml hEGF aqueous solution (1mg / ml, ratio of medicine to substance = 1: 100), ultrasonically mixed for about 4min to form a W / O emulsion, decompressed (vacuum degree 750mbar, temperature 35°C) to remove the organic solvent to form a gel-like substance, then added 1ml of phosphate buffer, and ultrasonically for 10min to obtain 1ml milky white uniform hEGF nano liposomes.
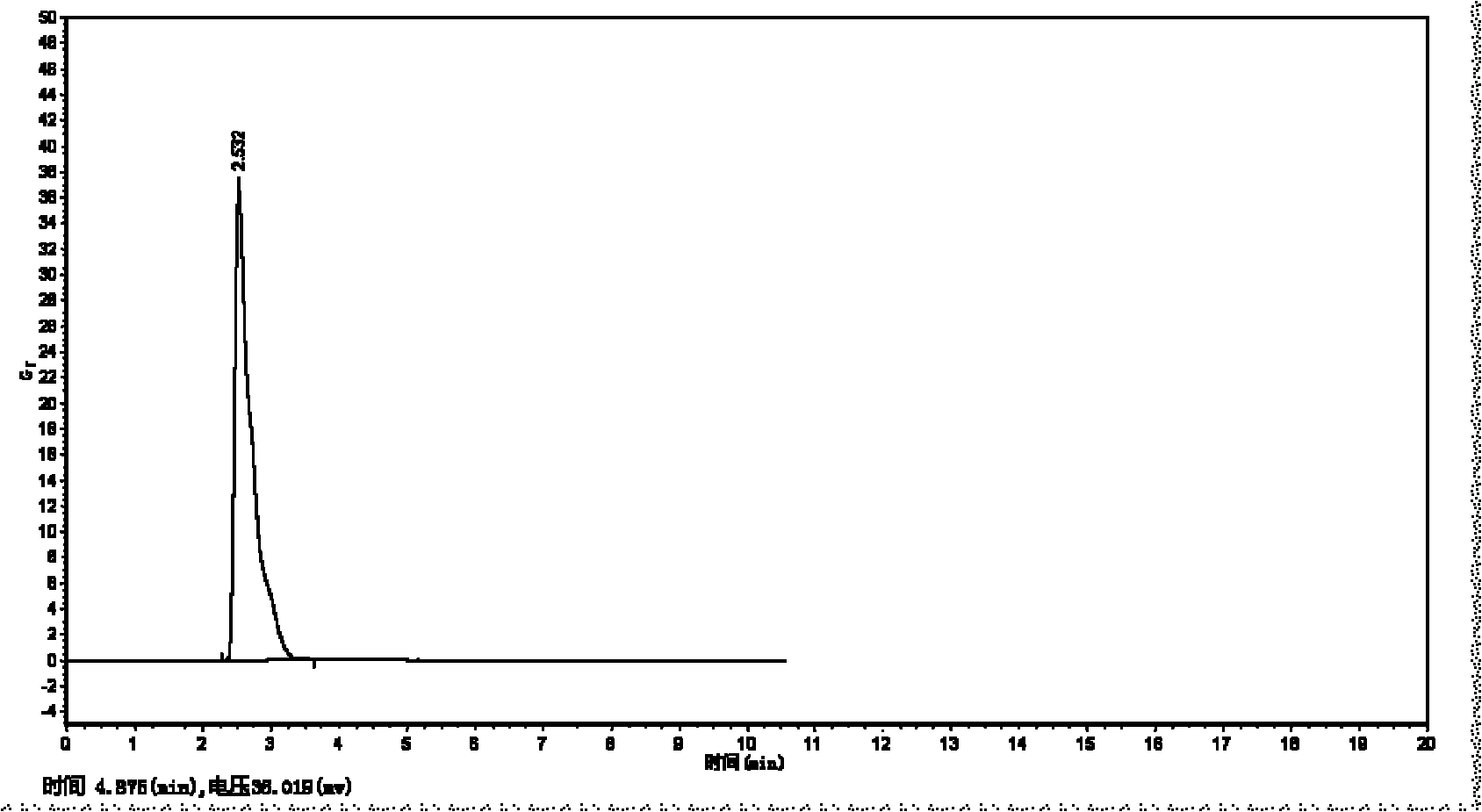
[0089] Preparation sample appearance: milky white homogeneous liqui...
Embodiment 3
[0093] Prescription three:
[0094] According to the prescription volume 1ml unit, the prescription is as follows:
[0095] Human Epidermal Growth Factor 1mg
[0096] Lecithin 89mg
[0097] Cholesterol (pharmaceutical grade) 11mg
[0098] Chloroform (analytical grade) 5ml
[0099] Phosphate buffer 1ml
[0100] Preparation process parameters:
[0101] Accurately weigh 89mg of lecithin, 11mg of cholesterol (PC: CHOL = 8: 1) dissolved in 5ml of chloroform ultrasonic (time 8min, frequency 53kHz) to dissolve, after mixing well, add 1ml hEGF aqueous solution (1mg / ml, drug substance ratio = 1: 100), ultrasonically mix for about 6 minutes to form a W / O emulsion, remove the organic solvent under reduced pressure (vacuum degree 800mbar, temperature 40°C) to form a gel-like substance, then add 1ml phosphate buffer (pH6.8), After ultrasonication for 10 min, 1 ml milky white homogeneous hEGF nanoliposomes were obtained.
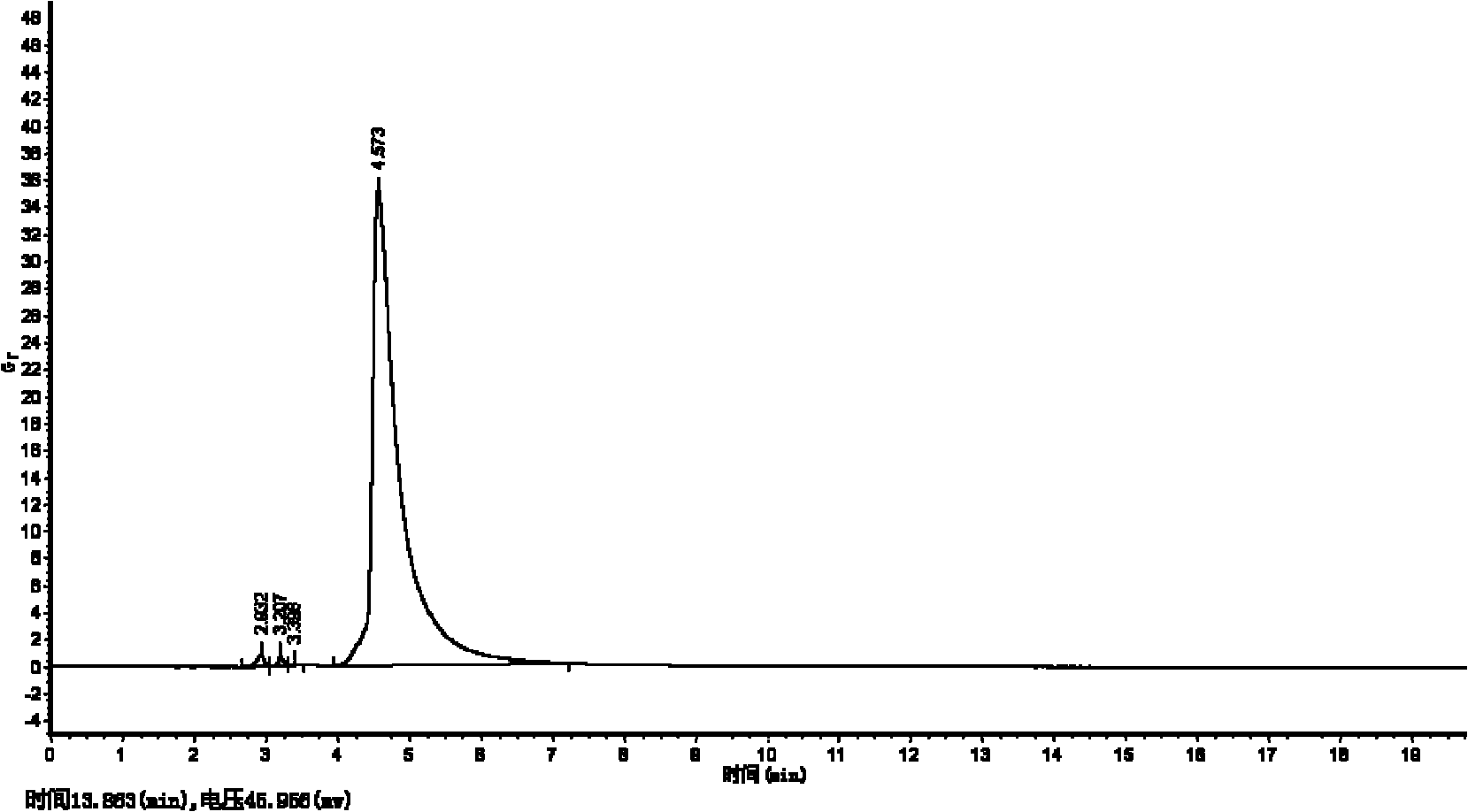
[0102] Encapsulation efficiency result: 31.38%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com