A kind of preparation method of lithium manganate battery material
A lithium manganate battery and manganese source technology, applied in the direction of manganate/permanganate, etc., can solve the problems of incomplete precipitation of lithium salt, difficulty in industrial production, and production of by-products, etc., to achieve uniform particle size and excellent performance , to avoid the effect of reunion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
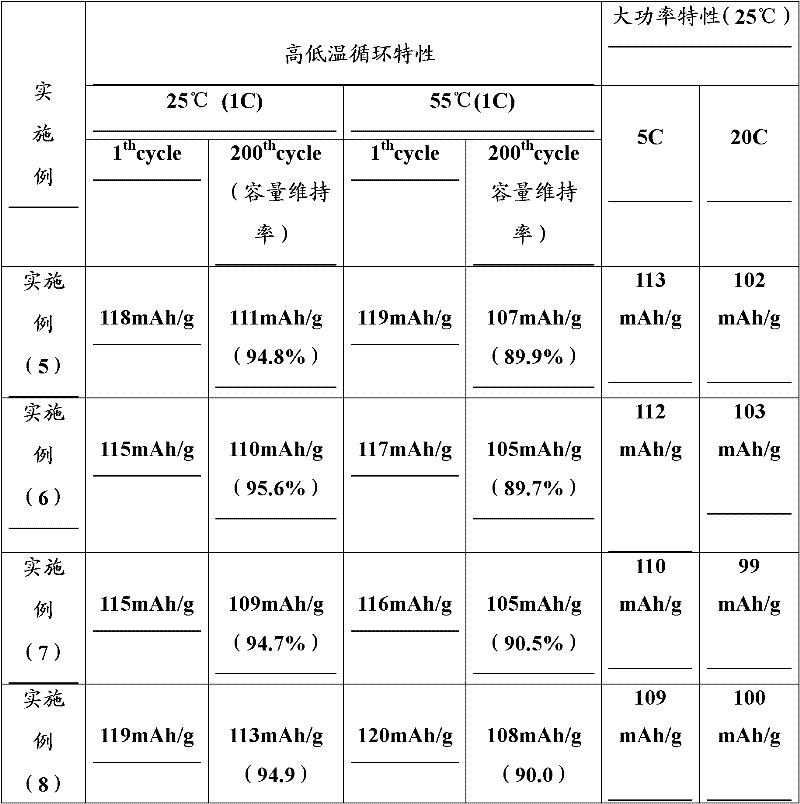
Examples
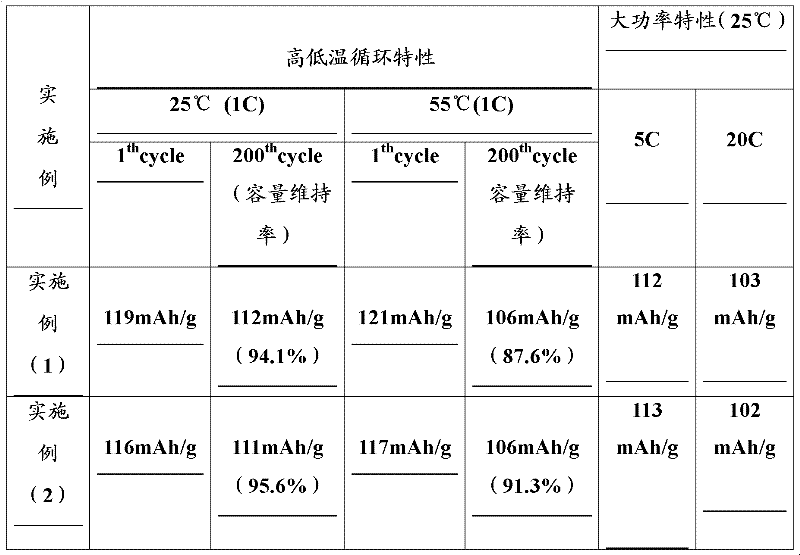
Embodiment (1
[0024] Manganese nitrate (2mol / L) and aluminum nitrate (1mol / L) were mixed according to (Mn:Al) molar number 99:1 and placed in a stainless steel reactor, and 4mol / L ammonia solution was added to the solution (the added volume was the original 25% of the solution) was stirred to give a precipitate. Continue to stir and heat the reaction solution to 70°C, and pass air and oxygen mixed gas (1:1) at a rate of 3L / min. When the pH value of the solution reaches about 6-7, stop the ventilation, let it stand, collect and use dilute hydrogen Aqueous lithium oxide solution (0.01 M) washed the precipitate. This precipitate is mixed with an appropriate amount (the amount of lithium hydroxide is 15% in excess of the stoichiometric ratio of the final product lithium manganate) 0.2M lithium hydroxide solution, heated to 200 ° C in a hydrothermal reactor, and reacted for 20 Hours. After the reaction is finished, add 1% (ratio to the amount of lithium manganate) sucrose solution into the rea...
Embodiment (2
[0026] Manganese nitrate (2mol / L) and cerium nitrate (1mol / L) were mixed according to (Mn:Ce) molar number 99:1 and placed in a stainless steel reactor, and 4mol / L ammonia solution was added to the solution (the added volume was the original 25% of the solution) was stirred to give a precipitate. Continue to stir and heat the reaction solution to 70°C, and pass air and oxygen mixed gas (1:1) at a rate of 3L / min. When the pH value of the solution reaches about 6-7, stop the ventilation, let it stand, collect and use dilute hydrogen Aqueous lithium oxide solution (0.01 M) washed the precipitate. This precipitate is mixed with an appropriate amount (the amount of lithium hydroxide is an excess of 25% compared with the stoichiometric ratio of the final product lithium manganate) 0.2M lithium hydroxide solution, heated to 300 ° C in a hydrothermal reactor, and reacted for 30 Hours. After the reaction is finished, add 1% (ratio to the amount of lithium manganate) sucrose solution ...
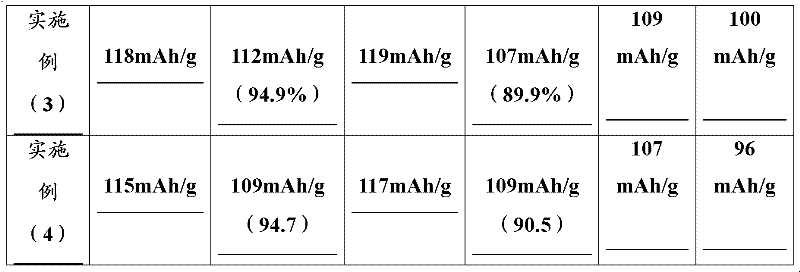
Embodiment (3
[0028] Manganese nitrate (2mol / L) and aluminum nitrate (1mol / L) were mixed according to (Mn:Al) molar number 99:1 and placed in a stainless steel reactor, and 4mol / L ammonia solution was added to the solution (the added volume was the original 25% of the solution) was stirred to give a precipitate. Continue to stir and heat the reaction solution to 70°C, and pass air and oxygen mixed gas (1:1) at a rate of 3L / min. When the pH value of the solution reaches about 6-7, stop the ventilation, let it stand, collect and use dilute hydrogen Aqueous lithium oxide solution (0.01 M) washed the precipitate. This precipitate is mixed with an appropriate amount (the amount of lithium hydroxide is 15% in excess of the stoichiometric ratio of the final product lithium manganate) 0.2M lithium hydroxide solution, heated to 200 ° C in a hydrothermal reactor, and reacted for 20 Hours. After the completion of the reaction, add 0.8% (ratio to the amount of lithium manganate) of SiO in the reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com