A kind of lithium nickel cobalt manganese oxide and preparation method thereof
A lithium-nickel-cobalt-manganese and oxide technology, which is applied in the field of preparation of lithium-nickel-cobalt-manganese oxide materials, can solve the problems that it is difficult to mix the precursor and lithium salt uniformly, and the mixing process takes time, so as to achieve uniform mixing of cations and save energy , the effect of high tap density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
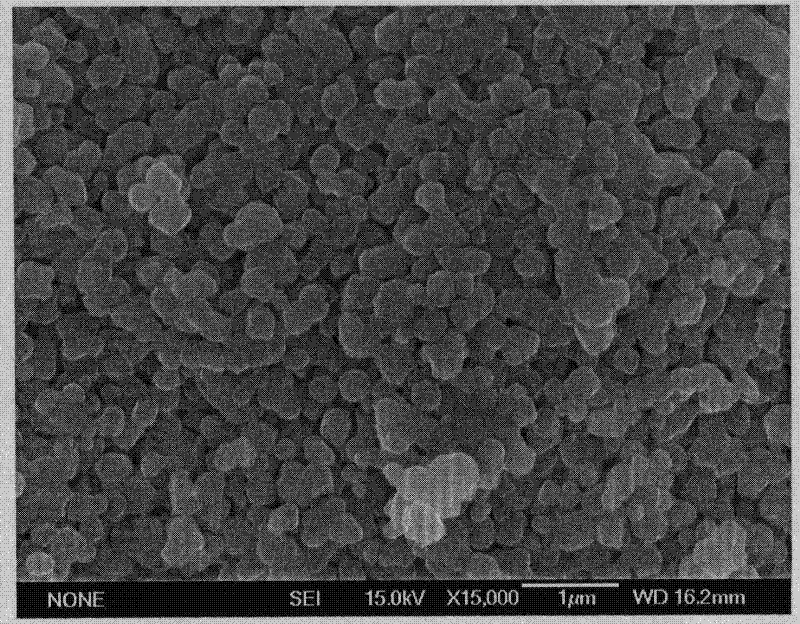
[0038] 0.11mol lithium acetate, 0.0333mol nickel acetate, 0.0333mol cobalt acetate, 0.0333mol manganese acetate are stirred and dissolved in dehydrated alcohol to obtain a concentration (sum of all cations) that is 1M (M is the concentration unit mol / L) solution (1 ). 0.21 mol of citric acid was stirred and dissolved in absolute ethanol to obtain a 2M solution (2). Slowly add solution (2) into solution (1), stir the resulting reaction mixture until a colloid is formed, then filter, put the filtered solid in an oven at 120°C, and dry for 4 hours to obtain a lithium nickel cobalt manganese oxide precursor body. The first sintering: After grinding the precursor, press the resulting material into a cylindrical shape at 2MPa, and put it into a muffle furnace to rise from room temperature to 450°C (the heating rate is 2°C / min), and keep it for 6 hours. Finish the first sintering, take out the material, grind it into fine powder, press it into a cylindrical shape under 2MPa, and pu...
Embodiment 2
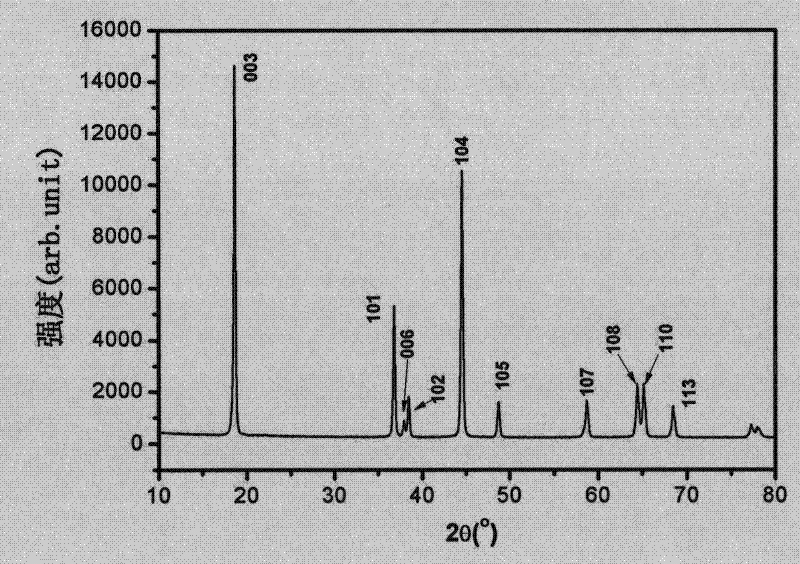
[0042] Change the second sintering time to be 18 hours, and other conditions are the same as in Example 1. The obtained products were analyzed by XRD, which showed that they were all lithium nickel cobalt manganese oxides, with sharp diffraction peaks, complete and good crystal form, and α-NaFeO 2 Structure, 006 / 102, 108 / 110 peaks are obvious, and the layered structure is good. At 25°C, 2.5-4.4V voltage range, charge and discharge at 0.1C, the first discharge capacity is 192mAh / g.
Embodiment 3
[0044] Change second sintering time to be 12 hours, other conditions are the same as embodiment 1. The obtained products were analyzed by XRD, showing that they were all lithium nickel cobalt manganese oxides without any miscellaneous peaks. The diffraction peak is sharp, the crystal form is complete and good, with α-NaFeO 2 Structure, 006 / 102, 108 / 110 peaks are obvious, and the layered structure is good. At 25°C, 2.5-4.4V voltage range, charge and discharge at 0.1C, the first discharge capacity is 190mAh / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com