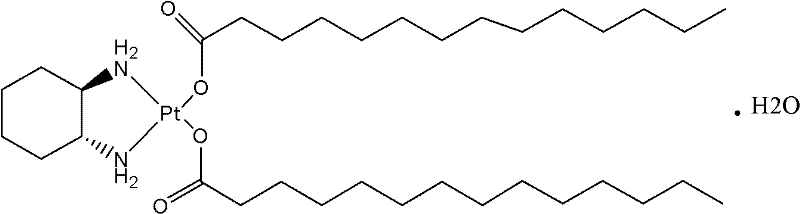
Milplatin freeze-dried powder injection and preparation method thereof
A technology of freeze-dried powder injection and miplatin, which is applied in the field of medicine, can solve the problems of increased cost and difficult work, and achieve the effects of reduced production cost, reduced production cost and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
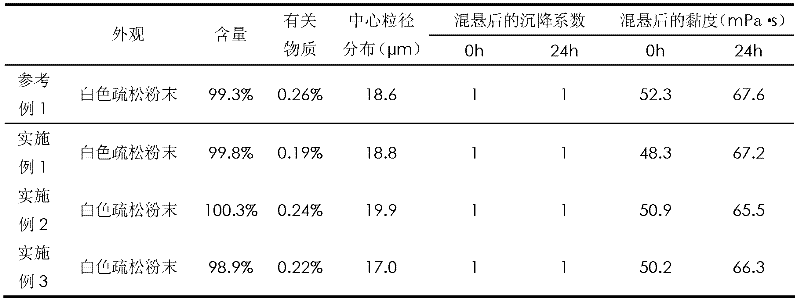
reference example 1
[0019] It can be prepared with reference to Chinese patent CN02818527.7:
[0020] prescription:
[0021] The preparation steps are as follows: measure tert-butanol, add it to the batching tank, and add the prescribed amount of rice platinum, stir the mixture at 30-40 ° C for 30 minutes, and use a Karl-Fischer hygrometer to measure the water content. The amount of water is 1.5mg / ml. Under sterile conditions, filter it with a 0.22μm organic microporous membrane until it is clear. Fill it into a vial with the amount calculated according to the intermediate, half-plug it with a butyl rubber stopper, and pack it into a plate. , into the freeze dryer, close the door, open the freeze dryer for freeze-drying, use heat transfer oil to cool the plate layer, make the product temperature reach -40 degrees, continue to freeze for 4 hours, and open the condenser until the temperature reaches -45 degrees Below, turn on the vacuum system again to make the vacuum of the front box below 133P...
Embodiment 1
[0023] prescription:
[0024] The preparation steps are as follows: measure tert-butanol, add it to the batching tank, control the solution temperature at 30-35°C, then weigh the prescribed amount of miplatin, stir or ultrasonically dissolve it evenly at the temperature, and then measure the prescribed amount Add dehydrated ethanol into the batching tank, stir evenly and maintain the solution temperature at 20-25°C. After the intermediate inspection is qualified, filter it with a 0.22μm organic microporous membrane under sterile conditions until it is clear, press The calculated amount of the intermediate is filled in a vial, half-plugged with a butyl rubber stopper, loaded into a plate, sent to a freeze dryer, closed the door, opened the freeze dryer for freeze-drying, and refrigerated the plates with heat transfer oil. Make the temperature of the product reach -40°C, continue to freeze for 4 hours, and at the same time turn on the condenser until the temperature reaches be...
Embodiment 2
[0026] prescription:
[0027] The preparation steps are as follows: measure tert-butanol, add it to the batching tank, control the solution temperature at 30-35°C, then weigh the prescribed amount of miplatin, stir or ultrasonically dissolve it evenly at the temperature, and then measure the prescribed amount Add dehydrated ethanol into the batching tank, stir evenly and maintain the solution temperature at 20-25°C. After the intermediate inspection is qualified, filter it with a 0.22μm organic microporous membrane under sterile conditions until it is clear, press The calculated amount of the intermediate is filled in a vial, half-plugged with a butyl rubber stopper, loaded into a plate, sent to a freeze dryer, closed the door, opened the freeze dryer for freeze-drying, and refrigerated the plates with heat transfer oil. Make the temperature of the product reach -40°C, continue to freeze for 4 hours, and at the same time turn on the condenser until the temperature reaches be...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com