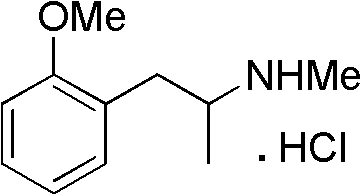
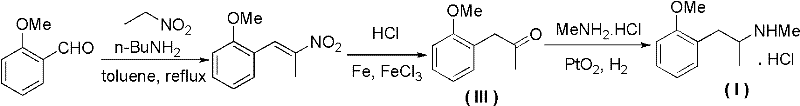
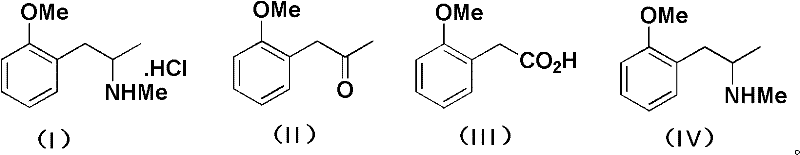
A kind of synthetic method of methoxyphenamine hydrochloride
A technology of methoxyphenamine hydrochloride and methoxybenzene, applied in chemical instruments and methods, preparation of organic compounds, preparation of amino hydroxyl compounds, etc., can solve problems such as unfavorable industrial production, difficult separation and purification, and large amount of manganese acetate , to achieve the effects of less three wastes, high reaction yield and low raw material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the preparation of o-methoxypropiophenone (III)
[0033] In a 1L three-necked flask, add 367g of acetic anhydride, 300g of o-methoxyphenylacetic acid, and 100g of pyridine in sequence, react at 40°C for 6 hours under nitrogen protection, remove excess anhydride under reduced pressure, add 400mL of 20% NaOH, stir, and then add Extract with 400mL of dichloromethane, wash the separated organic layer with 150mL of dilute hydrochloric acid and 200mL of pure water to remove pyridine, recover the dichloromethane under reduced pressure, distill the residue under reduced pressure, collect the fraction at 90-100℃ / 5mmHg, and obtain 197.3g O-methoxypropiophenone, yield 75%, GC purity 99.3%.
Embodiment 2
[0034] Embodiment 2: the preparation of o-methoxypropiophenone (III)
[0035] The mass ratio of the feed is o-methoxyphenylacetic acid: acetic anhydride: N-methylimidazole=1:0.5:0.4, the reaction temperature is 80°C, the reaction time is 5h, the organic solvent A is ethyl acetate, and the mass dosage is o-methoxyphenylacetic acid 2 times the quality of oxyphenylacetic acid, other operations are the same as in Example 1; the product o-methoxypropiophenone is obtained with a yield of 79.5% and a GC purity of 99.2%.
Embodiment 3
[0036] Embodiment 3: the preparation of o-methoxypropiophenone (III)
[0037]The mass ratio of the feed is o-methoxyphenylacetic acid: acetic anhydride: quinoline=1:1:0.6, the reaction temperature is 160°C, the reaction time is 1h, the organic solvent A is chloroform, and the mass dosage is the quality of o-methoxyphenylacetic acid 3 times, other operation is the same as embodiment 1; Obtain product o-methoxy Propiophenone, yield 72.5%, GC purity 98.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com