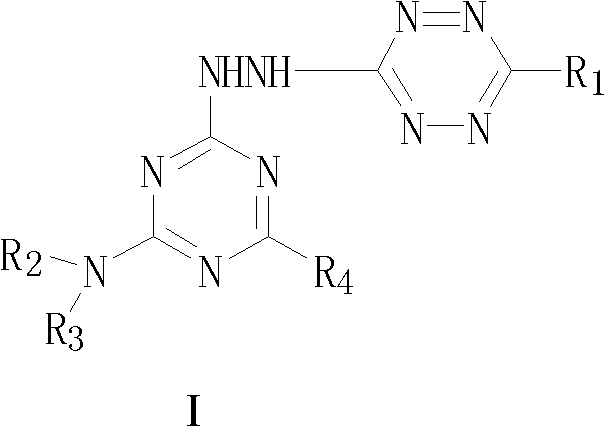
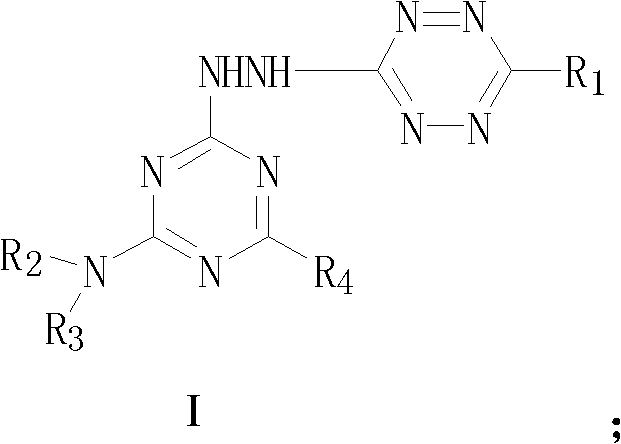
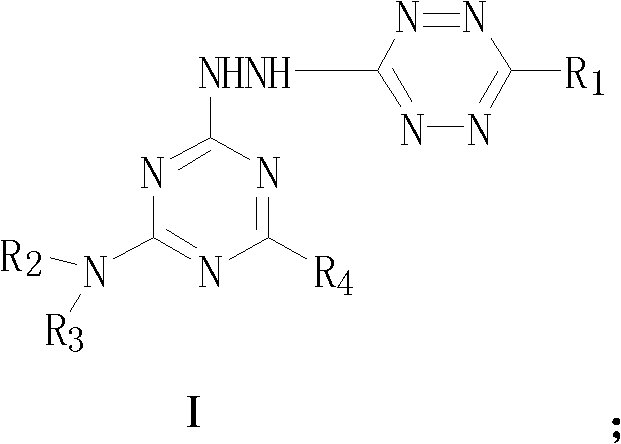
A kind of s-triazine derivative compound containing s-tetrazine ring and its preparation method
A compound, the technology of s-triazine, which is applied in the field of drug synthesis and medicine, can solve problems such as compounds that have not been reported on s-triazine derivatives, and achieve high biological activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 4-Chloro-6-(2-(6-(3,5-dimethyl-1H-pyrazol-1-yl)-1,2,4,5-tetrazin-3-yl)hydrazino)- Preparation of N-isobutyl-1,3,5-triazine-2-amine (Xu-1)
[0057]
[0058] Add 1.25 mmol of cyanuric chloride and 3.7 mmol of potassium carbonate into 30 ml of dioxane, stir, cool down to -5 ° C to 0 ° C, and start to slowly add the mixed solution (6-(3,5-dimethylpyridine) oxazol-1-yl)-3-hydrazine-1,2,4,5-tetrazine 1.25mmol dissolved in 20ml dioxane mixed solution), the reaction temperature was controlled to -5℃~3℃ during the dropwise addition, After the dropwise addition, the temperature was controlled at 2°C to 8°C, and the halogenation reaction was continued for 2 hours. After the reaction was completed, 2.5 mmol of isobutylamine was added to the reaction solution, and the temperature was heated to 100°C for reflux, and the reflux reaction was performed for 10 hours. , start cooling, suction filtration, remove the solid matter in the system, collect the filtrate, remove the solvent d...
Embodiment 2
[0065] 4-Chloro-6-(2-(6-(3,5-dimethyl-1H-pyrazol-1-yl)-1,2,4,5-tetrazin-3-yl)hydrazino)- Preparation of N-isopropyl-1,3,5-triazin-2-amine (Xu-2)
[0066]
[0067] Add 1.25 mmol of cyanuric chloride and 3.7 mmol of potassium carbonate into 30 ml of dioxane, stir, cool down to -5 °C to 0 °C, and start to slowly dropwise add 6-(3,5-dimethylpyrazole-1 - base)-3-hydrazino-1,2,4,5-tetrazine 1.25mmol dissolved in 20ml of dioxane mixed solution, control the reaction temperature during the dropwise addition -5 ℃ ~ 3 ℃, after the dropwise addition , control the temperature at 0 ℃ ~ 5 ℃, continue to stir the halogenation reaction for 1h, after the reaction is over, add 2.5mmol isopropylamine to the reaction solution, heat up to 100 ℃ reflux, reflux reaction for 10h, after the reaction is over, start cooling, pumping Filtration to remove the solid matter in the system, collect the filtrate, distill under reduced pressure to remove the solvent dioxane, after the distillation, add an ap...
Embodiment 3
[0074] 4-Chloro-6-(2-(6-morpholino-1,2,4,5-tetrazin-3-yl)hydrazino)-N,N-dipropyl-1,3,5-tri Preparation of oxazine-2-amine (Xu-3)
[0075]
[0076] Add 1.25mmol of cyanuric chloride and 3.7mmol of potassium carbonate to 30ml of dioxane, stir, cool down to -5℃~0℃, start to slowly dropwise add 6-morpholinyl-3-hydrazino-1,2 , 1.25mmol of 4,5-tetrazine is dissolved in a mixed solution of 20ml of dioxane, and the reaction temperature is controlled at -5℃~3℃ during the dropwise addition. Substitute the reaction for 2h, after the reaction is over, add 2.5mmol of di-n-propylamine to the reaction solution, heat up to reflux, reflux for 8h, after the reaction is over, start cooling, suction filtration, remove the solid matter in the system, collect the filtrate, and remove by distillation The solvent dioxane in the filtrate was removed, after the distillation was completed, an appropriate amount of ethyl acetate was added, and then a saturated aqueous sodium chloride solution was add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com