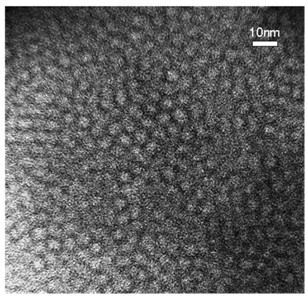
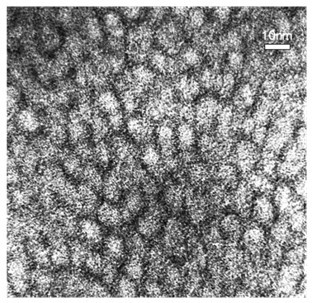
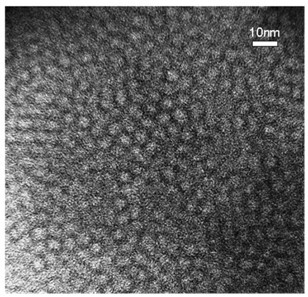
Dendritic grafted polycaprolactone
A technology of polycaprolactone and caprolactone, which is applied in the field of organic synthesis and biomedical materials, can solve the problems of unsatisfactory drug-loading capacity, poor affinity, and micelles that need to be improved, and achieve good application value and large drug-loading capacity , good micellar stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation of 4-(benzyloxyethyl) cyclohexanol: 22.32g (0.1921mol) of 4-hexanediol and 0.93g (0.0400mol) of chopped metal sodium were put in a round bottom flask, Under the protection of argon, heat to 120°C and stir with a magnet to disperse the sodium as much as possible. After 10 hours, it becomes a dark brown viscous liquid, and after being kept at 135°C for 12 hours, a dark brown lump is obtained. Cool to 50°C, inject about 70 mL of dry DMF to dissolve the brown lump. Keeping the temperature at 70°C, 12.29 g (0.0400 mol) of monobenzylethylene glycol p-toluenesulfonate dissolved in 16 mL of DMF was added dropwise with a syringe, and a total of about 92 mL of DMF was added. Stir and react at 70°C for 22h. After cooling to room temperature, filter under reduced pressure. The DMF in the filtrate was distilled off under reduced pressure and cooled to room temperature. Add about 60 mL of dichloromethane to precipitate excess cyclohexanediol, and filter under redu...
Embodiment 2
[0028] Preparation of 4-(benzyloxyethyl)cyclohexanone: In a 150mL flask, add 3.58g 4-(benzyloxyethoxy)cyclohexanol (0.0143mol) and 90mL CH 2 Cl 2 5.5 mL of Jones reagent was added dropwise while stirring, and the reaction was stirred at room temperature for 10 minutes after the addition was completed. After filtration, the filtrate was concentrated and purified by silica gel column chromatography to obtain 1.86 g of a colorless liquid with a yield of 52%. 1 H NMR (CDCl 3 , 300MHz, TMS): δ7.35-7.26(m, 5H), 4.59(s, 2H), 3.77-3.75(m, 1H), 3.71-3.65(m, 4H), 2.62-2.57(m, 2H) , 2.27-2.23(m, 2H), 2.12-2.08(m, 2H), 1.97-1.90(m, 2H). 13 C NMR (CDCl 3 , 75MHz,): δ211.7, 138.4, 128.6, 127.9, 73.5, 73.4, 69.9, 68.1, 37.4, 30.7.Anal.: Cald.for C 15 h 20 o 3 : C72.55, H 8.12; Found: C 72.14, H 8.36.
Embodiment 3
[0030] Preparation of 4-benzyloxyethoxy-ε-caprolactone (BECL): Add 2.09g of 70% mCPBA (8.47mmol) and magnetons in a 150mL round bottom flask, add 20mL CHCl 3 , and stirred until the mCPBA was completely dissolved. Add dropwise with 5mL CHCl 3 1.86 g (7.47 mmol) of dissolved 4-(benzyloxyethoxy) cyclohexanone was stirred for 5 hours. The solution in which a large amount of white crystals were precipitated was suction filtered, and the filtrate was collected and washed with 120mL saturated NaHCO 3 The filtrate was washed 3 times with distilled water, and the residue was concentrated and purified by silica gel column chromatography to obtain 1.66 g of a colorless liquid with a yield of 84%. 1 H NMR (CDCl 3 , 300MHz, TMS): δ7.38-7.27(m, 5H), 4.56(s, 2H), 4.50(dd, J 1 =10Hz,J 2 =13Hz, 1H), 4.04(dd, J 1 =6Hz,J 2 =13Hz, 1H), 3.72(br s, 1H), 3.63(s, 4H), 2.99(t, J=13Hz, 1H), 2.40(dd, J 1 =8.4Hz,J 2 =14.4Hz, 1H), 2.15-1.77(m, 4H). 13 C NMR (CDCl 3, 75MHz,): δ176.3, 138.4, 128...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average hydrated particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com