Preparation method of cathode material for lithium ion battery based on transition metal carbonate precursor
A lithium-ion battery and transition metal technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of complex process, commercial production of unfavorable materials, long reaction time, etc., and achieve narrow particle size distribution, high rate charge and discharge The effect of excellent performance and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Mix nickel nitrate, manganese nitrate, and urea according to the molar ratio of 1:3:10 to form a 250ml mixed solution. The concentration of nickel ions in the mixed solution is 1M. After thermal reaction for 12 hours, the precipitate was collected, washed and dried in vacuum to obtain the Ni0.25Mn0.75CO3 precursor. The resulting Ni 0.25 mn 0.75 CO 3 The precursor and lithium hydroxide were mixed at a molar ratio of 2:1, and then dry ball milled at 500 rpm with a ball-to-material ratio of 10:1 for 5 hours to obtain a powder.
[0027] The obtained powder is placed in a microwave air atmosphere furnace and sintered at a constant temperature of 550°C for 0.1h, then sintered at a constant temperature of 850°C for 0.5h, annealed at 600°C for 0.5h, and naturally cooled to room temperature to obtain a spinel transition Metal oxide material LiNi 0.5 mn 1.5 o 4 .
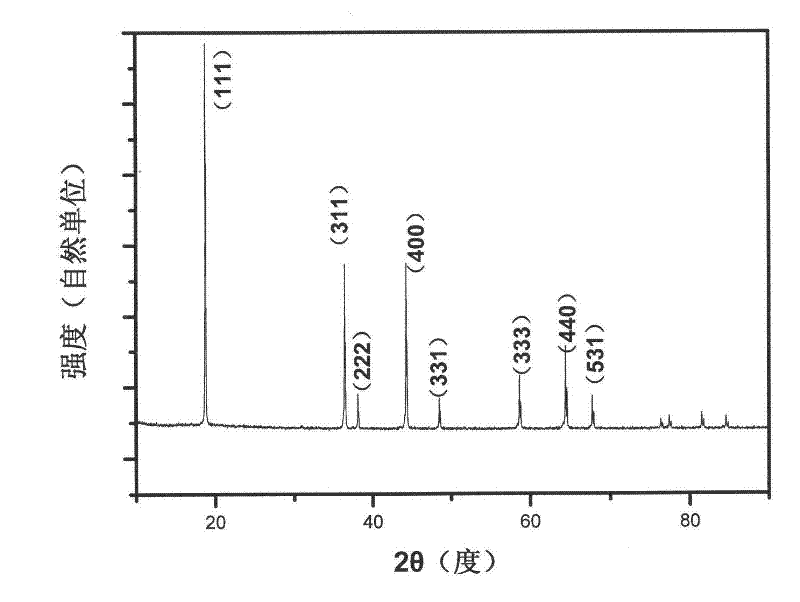
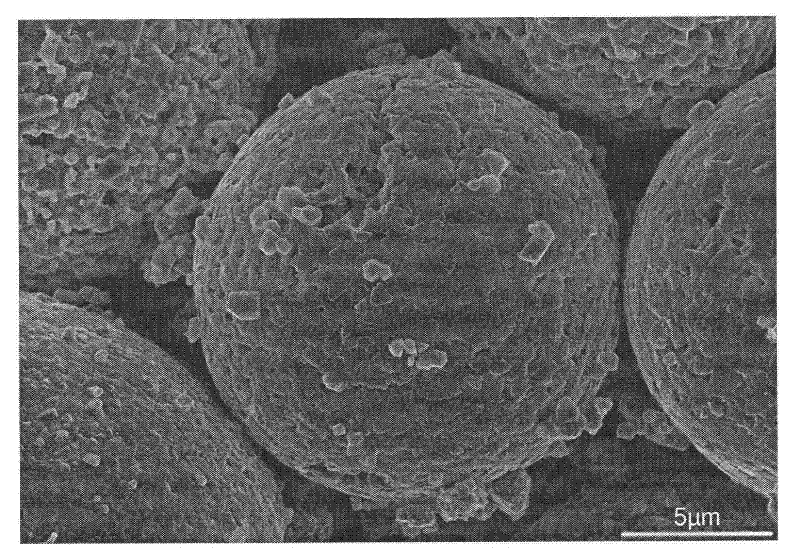
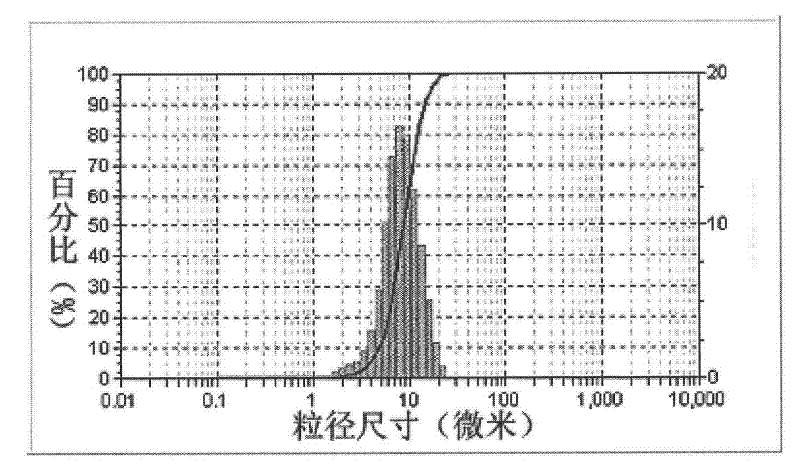
[0028] LiNi 0.5 mn 1.5 o 4 The X-ray diffraction pattern of figure 1 As shown, the scanning electron micr...
Embodiment 2
[0031] Nickel sulfate, manganese sulfate, and hexamethylenetetramine were mixed according to a molar ratio of 1:3:8 to form a 150ml solution. The concentration of nickel ions in the mixed solution was 1M, and the resulting solution was placed in a 50ml reactor at 180 Under the condition of ℃ hydrothermal reaction for 12h, the collected precipitate was washed and dried by rotary evaporation to obtain Ni 0.25 mn 0.75 CO 3 Precursor.
[0032] The resulting Ni 0.25 mn 0.75 CO 3The precursor and lithium carbonate were subjected to wet ball milling at a molar ratio of 1:1, the wet ball milling speed was 500 rpm, the ball milling time was 10 h, and the ball-to-material ratio was 10:1. Then the obtained powder was placed in a conventional resistance heating furnace for sintering at a constant temperature of 550°C for 2h, then sintered at a constant temperature of 850°C for 18h, annealed at 500°C for 8h, and naturally cooled to room temperature to obtain a spinel-type transition m...
Embodiment 3
[0034] Mix nickel sulfate, manganese sulfate, hexamethylene tetramonium, and sodium dodecylbenzenesulfonate in a molar ratio of 1:3:8:2 to form a 50ml solution. The concentration of nickel ions in the mixed solution is 2M , put the obtained solution in a 50ml reaction kettle for hydrothermal reaction at 180°C for 24 hours, collect the precipitate, wash and dry it in vacuum to prepare Ni 0.25 mn 0.75 CO 3 Precursor.
[0035] The resulting Ni 0.25 mn 0.75 CO 3 The precursor and lithium carbonate were mixed according to the stoichiometric ratio to form a 1M solution, and the resulting solution was dried with a high-speed centrifugal drying sprayer to obtain a mixed powder, and the feeding solution speed was 10ml / min; the gas flow rate of the nozzle was controlled by the pressure of compressed air. The pressure is controlled at 0.4MPa; the air inlet temperature is 200°C, and the outlet temperature is 80°C. The obtained powder was placed in a microwave air atmosphere furnace ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tap density | aaaaa | aaaaa |
| Discharge capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com