Method for synthesizing difluoroethanol
A technology of difluoroethanol and its synthesis method, which is applied in the fields of chemical instruments and methods, preparation of hydroxyl compounds, and preparation of organic compounds, etc., which can solve problems such as difficult difluoroethanol treatment process, difficult operation, and increased synthesis cost, and achieve convenient continuous Production operation, high synthesis yield and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
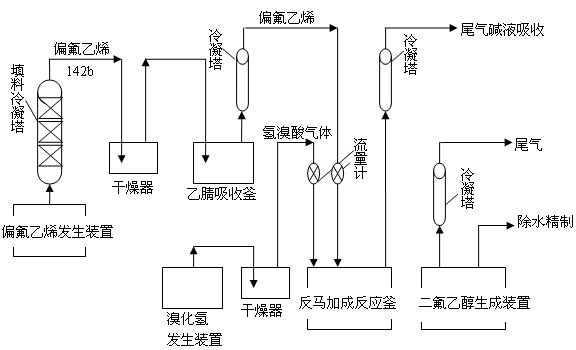
[0029] The steps of the synthetic method of difluoroethanol are as follows:
[0030] 1) In the reaction kettle, add a polar or weakly polar solvent and an initiator with a mass ratio of 300:1 to 30:1, heat to 50°C to 150°C, and continuously feed in a molar ratio of 1:1.05 to 1: 2.05% vinylidene fluoride gas and hydrobromic acid gas are reacted and distilled to obtain 1,1-difluoro-2-bromoethane, and the tail gas is absorbed with lye;
[0031] 2) In the reaction kettle, add 1,1-difluoro-2-bromoethane with a molar ratio of 2.5:1 to 1.2:1 and an aqueous sodium carbonate solution with a mass concentration of 10% to 30%, and stir the reaction under reflux conditions 2 hr to 5 hr, cool, add toluene, remove most of the water through azeotropic distillation of toluene and water, and then rectify to obtain difluoroethanol with a purity greater than 98%.
[0032] The polar or weakly polar solvent is N,N-dimethylformamide or acetonitrile. The used solvent is stable in property and good ...
Embodiment 1
[0045] 1) In a reaction kettle equipped with a gas inlet tube, a reflux condenser and a thermometer, add solvent acetonitrile and initiator azobisisobutyronitrile with a mass ratio of 40:1, and heat to 70°C; continuously feed in a molar ratio of 1: 1.2 Vinylidene fluoride gas and hydrogen bromide gas, react and distill to obtain 1,1-difluoro-2-bromoethane, and the tail gas is absorbed with lye.
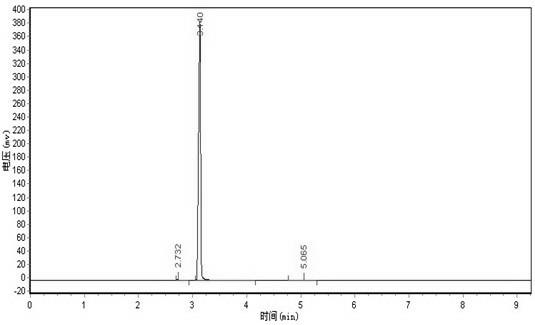
[0046] 2) In a reactor equipped with a stirrer, a reflux condenser and a thermometer, add 1,1-difluoro-2-bromoethane with a molar ratio of 2.5:1 and an aqueous solution of sodium carbonate with a mass concentration of 30%. The reaction was stirred under reflux for 2 hr. Cool, add toluene, remove most of the water through azeotropic distillation of toluene and water, and then rectify to obtain difluoroethanol with a purity of 98% and a yield of 95%.
Embodiment 2
[0048] 1) In a reaction kettle equipped with a gas inlet tube, a reflux condenser and a thermometer, add the solvent N,N-dimethylformamide and the initiator azobisisobutyronitrile with a mass ratio of 100:1, and heat to 100 ℃; Continuously feed vinylidene fluoride gas and hydrogen bromide gas with a molar ratio of 1:1.1 for reaction and distillation to obtain 1,1-difluoro-2-bromoethane, and the tail gas is absorbed with lye.
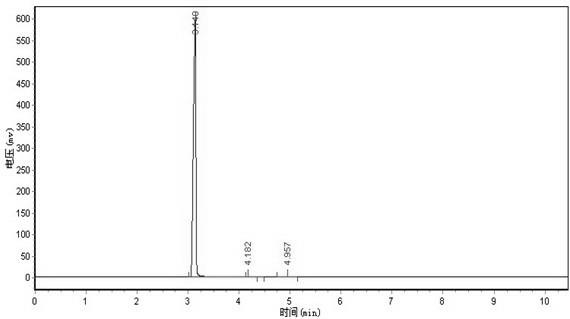
[0049] 2) In a reactor equipped with a stirrer, a reflux condenser and a thermometer, add 1,1-difluoro-2-bromoethane with a molar ratio of 2:1 and an aqueous solution of sodium carbonate with a mass concentration of 20%. The reaction was stirred under reflux for 5 hr. Cool, add toluene, remove most of the water through azeotropic distillation of toluene and water, and then rectify to obtain difluoroethanol with a purity of 98.5% and a yield of 92%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com