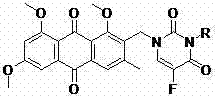
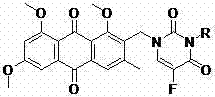
Emodin and fluorouracil combined compound having antitumor activity and preparation method thereof
A technology with anti-tumor activity and fluorouracil, which is applied in the field of emodin and 5-fluorouracil compound and its preparation, can solve the problems of lack of selectivity between tumor cells and normal cells, high cytotoxicity, and large toxic and side effects, so as to improve the therapeutic dose Close to the toxic dose, enrich the structure-activity relationship, and reduce toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
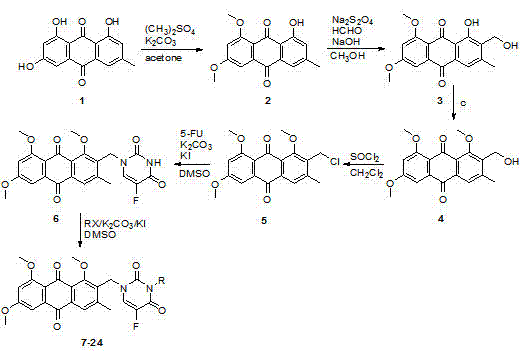
[0034] Example 1: 1-hydroxy-6,8-dimethoxy-3-methyl-9,10-anthraquinone 2
[0035] Emodin (4.05 g, 0.015 mol) was dissolved in acetone (150 ml), potassium carbonate (8.28 g, 0.06 mol) was added, dimethyl sulfate (4.0 ml) was added under stirring, and refluxed for 5 h. Acetone was distilled off, water was added, extracted with dichloromethane, dried over anhydrous sodium sulfate, and separated by column chromatography (dichloromethane) to obtain 3.17 g of a yellow solid with a yield of 71%. 1 H NMR (400 MHz, CDCl 3 ) δ7.58 (s, 1H), 7.47 (d, J = 2.4 Hz, 1H), 7.08 (s, 1H), 6.79 (d, J = 2.4 Hz, 1H), 4.03 (s, 3H), 3.99 (s, 3H), 2.43 (s, 3H).
Embodiment 2
[0036] Example 2: 1-hydroxy-6,8-dimethoxy-2-hydroxymethyl-3-methyl-9,10-anthraquinone 3
[0037] 1-Hydroxy-6,8-dimethoxy-3-methyl-9,10-anthraquinone 2 (149 mg, 0.5 mmol) was dissolved in methanol (75 ml), and sodium hydroxide (1 M, 0.5 ml). Then, the reaction flask was cooled in an ice-water bath, protected by nitrogen, and an aqueous solution of sodium dithionite (131 mg, 0.75 mmol) was added via a syringe, and 37% aqueous formaldehyde (1 mL) was added after 5 minutes. After 30 minutes, the reaction solution was poured into ice water containing 3% hydrogen peroxide. After oxidizing for 30 minutes, acidify to weak acidity with hydrochloric acid. Extracted with dichloromethane, dried over anhydrous sodium sulfate, and separated by column chromatography (dichloromethane: methanol = 100: 1) to obtain 135 mg of a yellow solid with a yield of 82%. 1 H NMR (400 MHz, DMSO- d 6 ) δ13.66 (s, 1H), 7.38 (s, 1H), 7.18 (s, 1H), 6.93 (s, 1H), 4.94 (t, J = 5.2 Hz, 1H), 4.55 (d, J = ...
Embodiment 3
[0038] Example 3: 1,6,8-trimethoxy-2-hydroxymethyl-3-methyl-9,10-anthraquinone 4
[0039] 1-Hydroxy-6,8-dimethoxy-2-hydroxymethyl-3-methyl-9,10-anthraquinone 3 (656 mg, 2.0 mmol) was dissolved in acetone (50 ml) and potassium carbonate was added (1.38 g, 10 mmol), and dimethyl sulfate (0.38 mL, 4.0 mmol) was added dropwise with stirring. After refluxing for 16 hours, the acetone was distilled off under reduced pressure. Add water, extract with dichloromethane, dry over anhydrous sodium sulfate, and separate by column chromatography (dichloromethane: methanol = 100: 1) to obtain 554 mg of a yellow solid with a yield of 81%. 1 H NMR (400 MHz, CDCl 3 ) δ7.84 (s, 1H), 7.34 (d, J = 2.0 Hz, 1H), 6.78 (d, J = 2.0 Hz, 1H), 4.82 (s, 2H), 4.02 (s, 3H), 3.99 (s, 3H), 3.97 (s, 3H), 2.53 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com