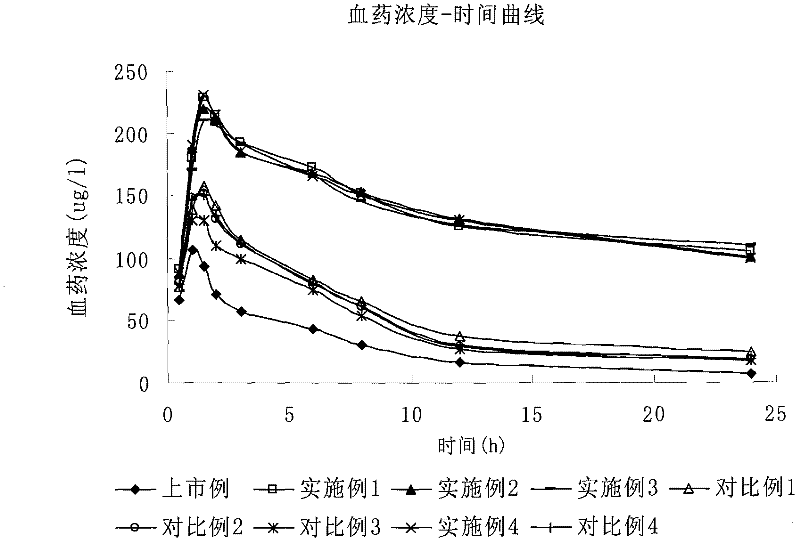
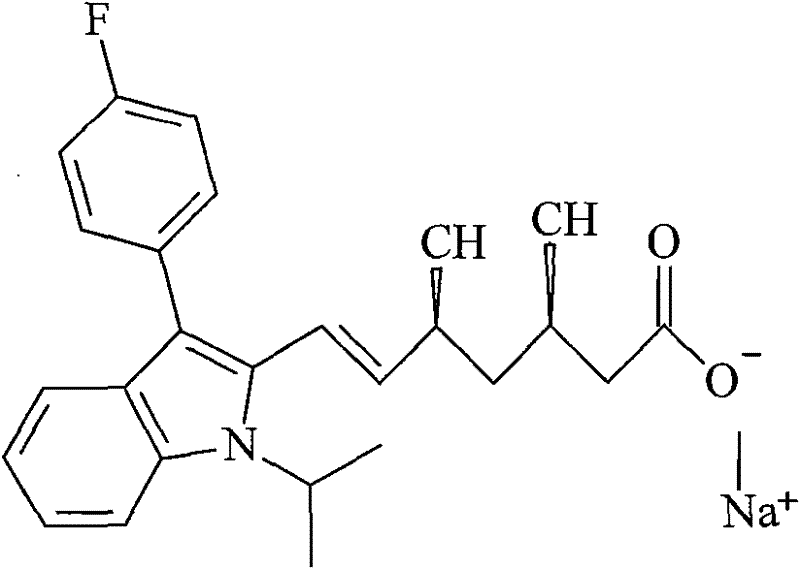
Fluvastatin sodium liposome solid preparation
A technology of fluvastatin sodium and solid preparation, applied in the field of pharmaceutical preparations, can solve problems such as increasing drug retention time, and achieve the effects of improving medication compliance, reducing the number of administrations, and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The preparation of embodiment 1 fluvastatin sodium liposome capsule
[0058] The raw and auxiliary materials used are as follows:
[0059]
[0060] Adopt the following production process to prepare fluvastatin sodium liposome capsules:
[0061] (1) 30g soybean lecithin, 20g cholesterol, 4g soybean sterol, 10g sorbitan stearate are dissolved in 200ml volume ratio and are the mixed solvent of acetone and ethanol of 2: 3, mix uniformly, in rotary evaporator Remove the organic solvent under reduced pressure to obtain a phospholipid film;
[0062] (2) Add 200ml of phosphoric acid-sodium hydrogen phosphate buffer solution with a pH of 7.0, shake and stir to completely hydrate the phospholipid membrane, homogeneously emulsify at 3000rpm, and filter through a microporous membrane to obtain a blank liposome suspension;
[0063] (3) Dissolve 20 g of fluvastatin sodium in the blank liposome suspension, filter through a 0.45 μm microporous membrane, incubate at 49° C. for 50...
Embodiment 2
[0066] The preparation of embodiment 2 fluvastatin sodium liposome capsules
[0067] The raw and auxiliary materials used are as follows:
[0068]
[0069] Adopt the following production process to prepare fluvastatin sodium liposome capsules:
[0070] (1) 20g soybean lecithin, 18g cholesterol, 6g soybean sterol, 10g sorbitan stearate are dissolved in 20ml volume ratio and are the mixed solvent of acetone and ethanol of 2: 3, mix uniformly, in rotary evaporator Remove the organic solvent under reduced pressure to obtain a phospholipid film;
[0071] (2) Add 200ml of phosphoric acid-sodium hydrogen phosphate buffer solution with a pH of 7.0, shake and stir to completely hydrate the phospholipid membrane, homogeneously emulsify at 3000rpm, and filter through a microporous membrane to obtain a blank liposome suspension;
[0072] (3) 20 g of fluvastatin sodium was dissolved in blank liposome suspension, filtered through a 0.45 μm microporous membrane, incubated at 55° C. fo...
Embodiment 3
[0075] The preparation of embodiment 3 fluvastatin sodium liposome capsules
[0076] The raw and auxiliary materials used are as follows:
[0077]
[0078] Adopt the following production process to prepare fluvastatin sodium liposome capsules:
[0079] (1) 40g soybean lecithin, 36g cholesterol, 12g soybean sterol and 22g sorbitan stearate are dissolved in 40ml volume ratio and are the mixed solvent of acetone and ethanol of 2: 3, mix homogeneously, in rotary evaporator Remove the organic solvent under reduced pressure to obtain a phospholipid film;
[0080] (2) Add 400 ml of phosphoric acid-sodium hydrogen phosphate buffer solution with a pH of 7.0, shake and stir to completely hydrate the phospholipid membrane, homogeneously emulsify at 300 rpm, and filter through a microporous membrane to obtain a blank liposome suspension;
[0081] (3) 40 g of fluvastatin sodium was dissolved in blank liposome suspension, filtered through a 0.45 μm microporous membrane, kept at 40° C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com