Hydrothermal synthesis preparation method of ion doping high-performance lithium iron phosphate
A lithium iron phosphate, ion doping technology, applied in electrical components, battery electrodes, circuits, etc., can solve the problems of unsatisfactory lithium ion diffusion rate, uneven particle distribution, poor high rate performance, etc., and achieve excellent electrochemical performance. , The process control is simple, the crystallinity is good
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
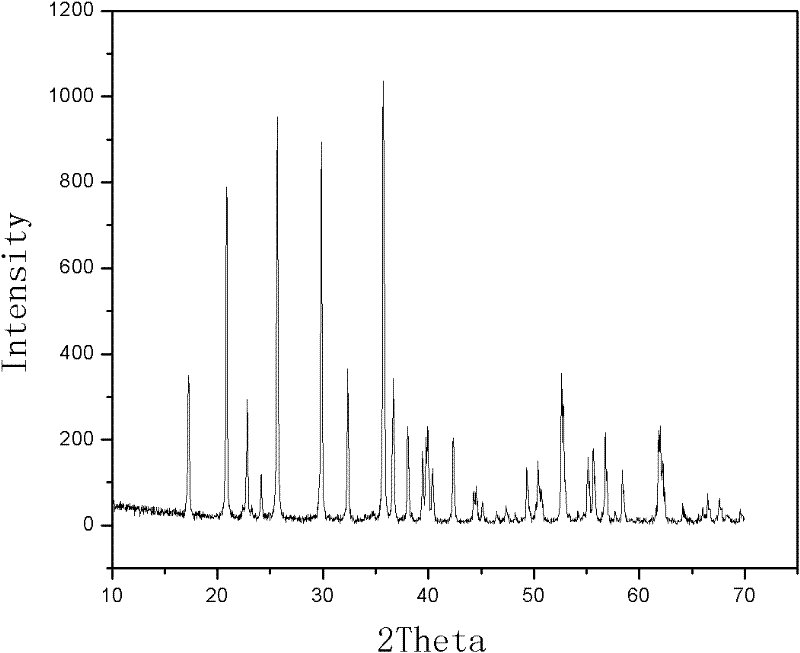
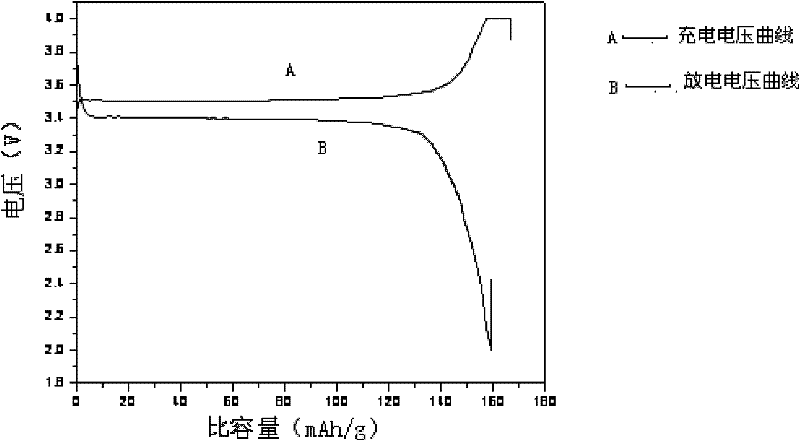
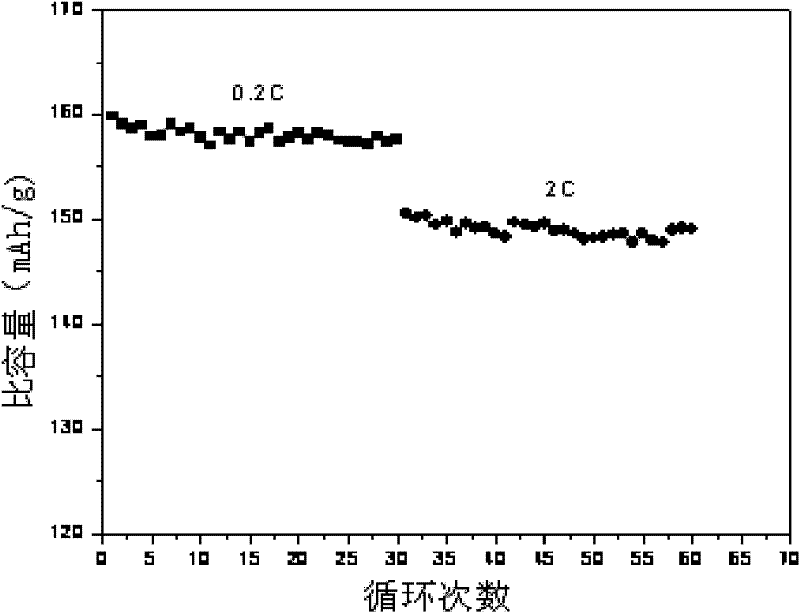
[0022] a): see Figure 1-Figure 3 , the 1021.65g FeSO 4 ·7H 2 O, 9.73gNiCl 2 , 100g of ascorbic acid and 432.5g of 85% H 3 PO 4 Mix together, add 5.925g of polyvinyl alcohol, then add 2.5L of deionized water, and then keep stirring,
[0023] At the same time, 472gLiOH·H 2 O (analytical pure) was dissolved in 2.5L of deionized water and stirred to obtain a lithium oxide solution, and then the lithium hydroxide solution was slowly added dropwise to the mixed solution to adjust the pH value to 8. Then the mixed solution was transferred to a 10L reaction kettle, deionized water was added appropriately, sealed, and kept at 180° C. for 2 hours.
[0024] b): After the above reaction is completed, cool to room temperature, filter and wash the product, and dry the filter cake in a vacuum at 80° C. for 12 hours to obtain nickel-containing lithium iron phosphate powder.
[0025] c): The nickel-containing lithium iron phosphate powder and maleic acid are ball-milled in an alcohol s...
Embodiment 2
[0027] a): 457.2g of FeCl 2 , 19.46gNiCl 2 , 98.8g of maleic acid and 432.5g of 85% H 3 PO 4 Mix together, add 8.89g of polyacrylamide, then add 2.5L of deionized water, and then keep stirring. At the same time, 742.5g of lithium acetate (analytical pure) was dissolved in 2.5L of deionized water, and stirred to obtain a pure solution. Then the lithium acetate solution was slowly added dropwise to the mixed solution to adjust the pH value to slightly alkaline. Then the mixed solution was transferred to a 10L reaction kettle, deionized water was added appropriately, sealed, and kept at 190° C. for 2 hours.
[0028] b): After the above reaction is completed, cool to room temperature, filter and wash the product, and dry the filter cake in a vacuum at 90° C. for 12 hours to obtain nickel-containing lithium iron phosphate powder.
[0029] c): The nickel-containing lithium iron phosphate powder and citric acid are ball-milled in an alcohol solution at a mass ratio of 10:3 for...
Embodiment 3
[0031] a): 409.93g of iron acetate, 8.84g of nickel acetate, 100g of ascorbic acid and 431.25 of NH 4 h 2 PO 4 Mix together, add a mixture of 11.85 polyvinyl alcohol and polyacrylamide, then add 2.5 L of deionized water, and then keep stirring. At the same time, 472gLiOH·H 2 O (analytically pure) was dissolved in 2.5L deionized water, stirred to obtain a pure solution, and then slowly added dropwise to the mixed solution to adjust the pH value to weakly alkaline. Then the mixed solution was transferred to a 10L reaction kettle, deionized water was added appropriately, sealed, and kept at 200°C for 1.5h.
[0032] b): After the above reaction is completed, cool to room temperature, filter and wash the product, and dry the filter cake in a vacuum at 95° C. for 12 hours to obtain nickel-containing lithium iron phosphate powder.
[0033] c): The nickel-containing lithium iron phosphate powder and citric acid are ball-milled in an alcohol solution at a mass ratio of 5:1 for 2 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge specific capacity | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com