Lithium-rich manganese-based cathode material precursor, cathode material and preparation method thereof
A positive electrode material, lithium-rich manganese-based technology, applied in the field of positive electrode materials and their preparation, lithium-rich manganese-based positive electrode material precursors, can solve the problems of many synthesis steps, complex process conditions, difficult particle size control, etc., to achieve repeatable Good performance, uniform product quality, and good layered crystal structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] A preparation method of a lithium-rich manganese-based cathode material precursor, comprising the steps of:
[0050] Step 1. Preparation of metal salt solution: mix soluble manganese salt, cobalt salt, nickel salt (ie metal salt) and surfactant with water in a certain proportion, stir and dissolve to obtain metal salt solution.
[0051] Above-mentioned manganese salt is: one or more in manganese nitrate, manganese acetate, manganese chloride and manganese sulfate;
[0052] Above-mentioned nickel salt is: one or more in nickel nitrate, nickel acetate, nickel chloride and nickel sulfate;
[0053] The above cobalt salts are: one or more of cobalt nitrate, cobalt acetate, cobalt chloride and cobalt sulfate.
[0054] In the metal salt solution, metal cations are composed of nickel ions, cobalt ions and manganese ions, and the total concentration is 0.01mol / L-2.0mol / L (for example, it can be 0.01mol / L, 0.05mol / L, 0.1mol / L, 0.2mol / L, 0.4mol / L, 0.5mol / L, 0.6mol / L, 1.0mol / L, ...
Embodiment 1
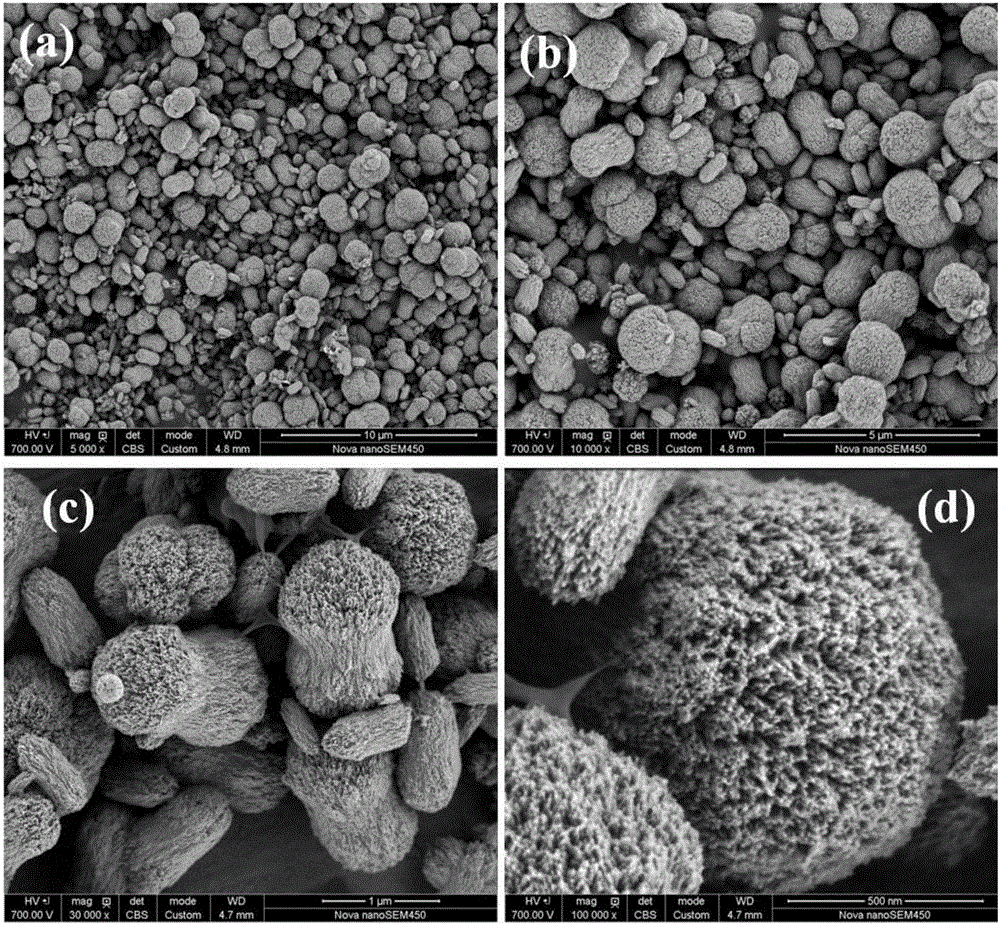
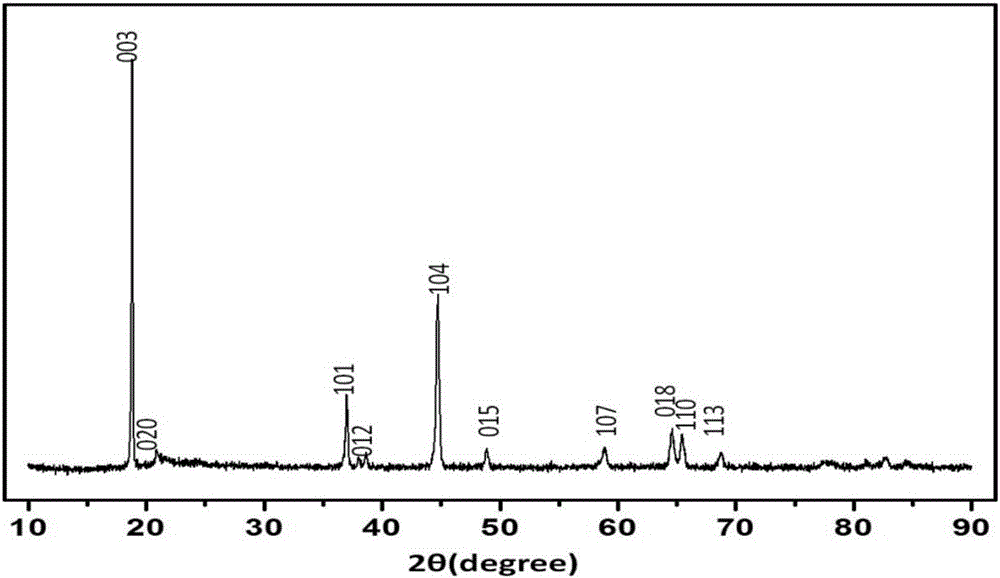
[0083] A lithium-rich manganese-based positive electrode material precursor and positive electrode material were prepared using a high gravity method. The lithium-rich manganese-based material precursor prepared in this example has a molecular formula of Mn 0.4 Ni 0.24 co 0.16 (CO 3 ) 0.8 ; The chemical formula of the lithium-rich manganese-based positive electrode material prepared by the present embodiment is Li 1.2 mn 0.4 Ni 0.24 co 0.16 o 2 , the specific preparation method is as follows:
[0084] (1), preparation of metal salt solution: take respectively the manganese nitrate of 0.2mol, the nickel nitrate of 0.12mol, the cobalt nitrate of 0.08mol, the polyvinylpyrrolidone (PVP) of 0.04mol, be dissolved in ultrapure water, be mixed with 2 liter of solution to obtain a 0.2mol / L metal salt solution.
[0085] (2), preparation of precipitant solution: take by weighing 0.42mol of sodium carbonate, be dissolved in ultrapure water, be mixed with 2 liters of aqueous solu...
Embodiment 2
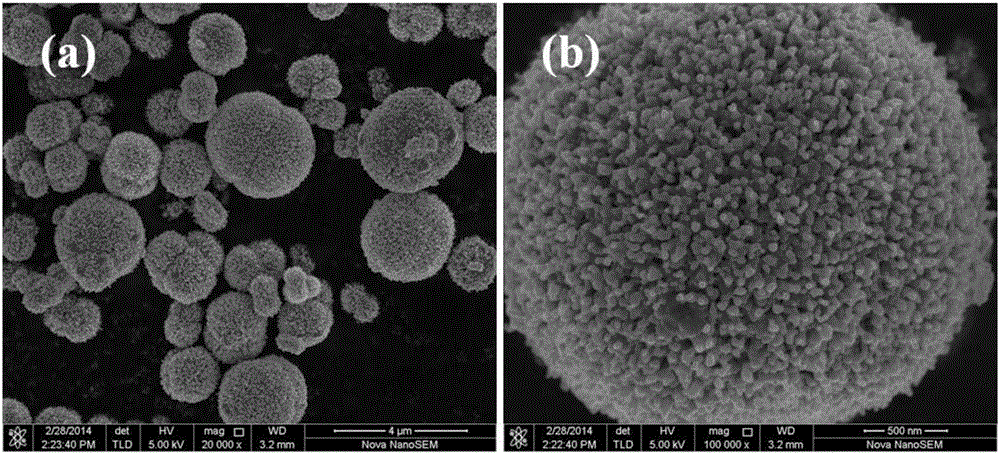
[0096] The precursor of lithium-rich manganese-based materials was prepared by using the hypergravity method. The lithium-rich manganese-based material precursor prepared in this example has a molecular formula of Mn 0.58 Ni 0.18 co 0.04 (OH) 1.6 ; The expression of the lithium-rich manganese-based positive electrode material prepared by the present embodiment is Li 1.2 mn 0.4 Ni 0.24 co 0.16 o 2 , the specific method is as follows:
[0097] (1) Preparation of metal salt solution: take respectively the manganese nitrate of 0.725mol, the nickel nitrate of 0.225mol, the cobalt nitrate of 0.05mol, the PVP of 0.08mol, be dissolved in ultrapure water, be mixed with the solution of 1 liter, thus obtain 1mol / L metal salt solution.
[0098] (2) Precipitant solution preparation: Weigh 2.1 mol of sodium hydroxide, dissolve in ultrapure water, and prepare 1 liter of aqueous solution to obtain a precipitant solution with a concentration of 2.1 mol / L.
[0099] (3) Precursor prepa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com