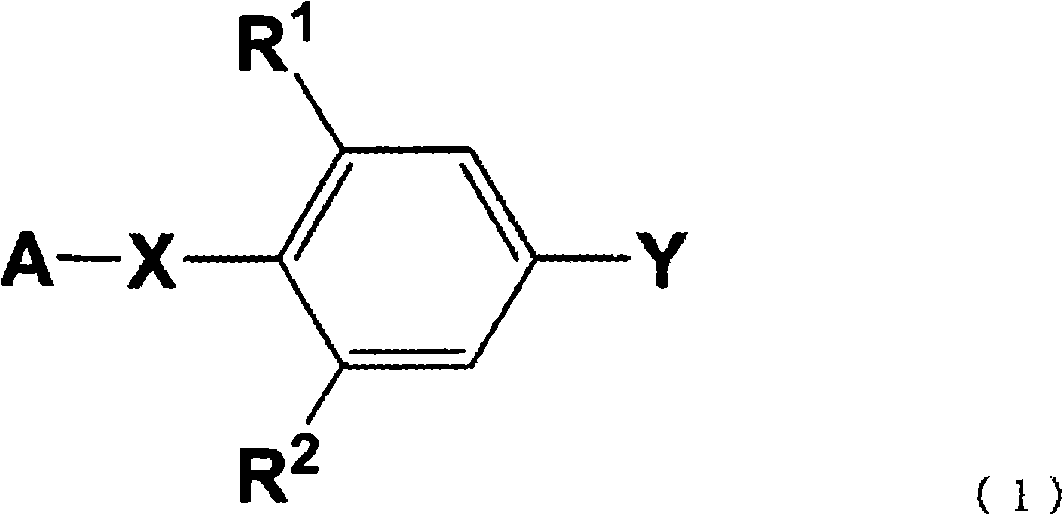
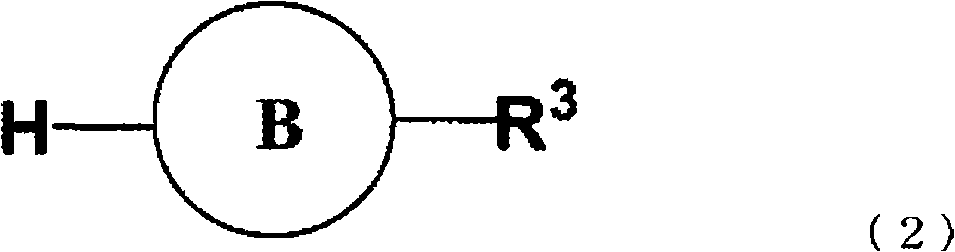
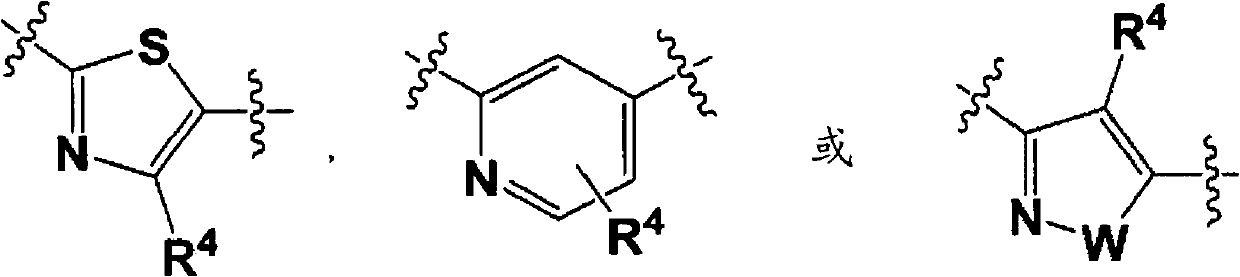
Process for producing phenyl-substituted heterocyclic derivative through coupling using transition metal catalyst
一种制造方法、苯基取代的技术,应用在杂环化合物有效成分、散装化学品生产、含有效成分的医用配制品等方向,能够解决苯基取代杂环衍生物没有报道等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0255] Hereinafter, the present invention will be specifically described by way of examples and the like. However, it does not mean that the scope of the present invention is limited by these examples.
[0256] In this example, the following equipment and the like were used for analysis and purification.
[0257] TLC: E. Merck Silicone 60F 254 (0.25mm)
[0258] Flash column chromatography: Biotage Flash, Si40
[0259] Preparative thin-layer chromatography (PTLC): Merck silica gel 60F 254 (1mm)
[0260] Liquid Chromatography / Mass Spectrometry (LC / MS)
[0261] Analysis system: SHIMAZU LCMS-2010A
[0262] Software: LCMS Solution
[0263] Experimental conditions:
[0264] Column: Phenomenex Gemini 3μm 4.6mm×30mm
[0265] Flow rate: 1.2mL / min
[0266] Measuring temperature: 40°C
[0267] A Solvent: 5%MeCN / 95%H 2 O+0.05% TFA
[0268] B Solvent: 95% MeCN / 5% H 2 O+0.05% TFA
[0269] MS mode: ESI+
[0270] ESI voltage: 4.5KV
[0271] Source temperature: 130°C
[0272] D...
reference example 1
[0280] Synthesis of tert-butyl 4-methylthiazole-5-carboxylate
[0281]
[0282] A mixture of 4-methyl-5-thiazolecarboxylic acid (1.36 g, 9.48 mmol) and thionyl chloride (28.7 mL) was stirred at 80° C. for 1 hour. The reaction solution was concentrated under reduced pressure to remove thionyl chloride, and the resulting crude product was dried under reduced pressure. To a dichloromethane (5.68 mL) solution of the crude product were added tert-butanol (2.84 mL) and pyridine (16.9 mL), followed by stirring at 60° C. overnight. After the reaction was completed, the reaction solution was concentrated under reduced pressure, and saturated aqueous sodium carbonate solution and ethyl acetate were added to the obtained crude product. After separating ethyl acetate, ethyl acetate was added to saturated aqueous sodium bicarbonate solution for extraction. The combined organic phases were washed with saturated brine and dried over anhydrous magnesium sulfate. After magnesium sulfate ...
reference example 2
[0285] Synthesis of 5-iodo-2-isobutoxybenzonitrile
[0286]
[0287] A solution of 2-methyl-1-propanol (0.56 mL, 6.06 mmol) in N,N-dimethylformamide (10 mL) was cooled to 0° C. and sodium hydride (242 mg, 60% in mineral oil) was added in small portions suspension, 6.06 mmol). After stirring the suspended reaction liquid at 0°C for 5 minutes, the temperature was raised to 23°C, stirred at room temperature for 10 minutes, and cooled to 0°C again. 2-Fluoro-5-iodobenzonitrile (1.0 g, 4.04 mmol) was added to the reaction liquid, and the reaction liquid was warmed up to room temperature, followed by stirring for 1.5 hours. After the reaction, water (20 mL) was added to the reaction liquid, and extracted with ethyl acetate (3×30 mL). The organic phases were combined, washed with saturated brine (3×30 mL), and dried over anhydrous magnesium sulfate. After magnesium sulfate was filtered off, the solvent was concentrated under reduced pressure, and the obtained crude product was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com