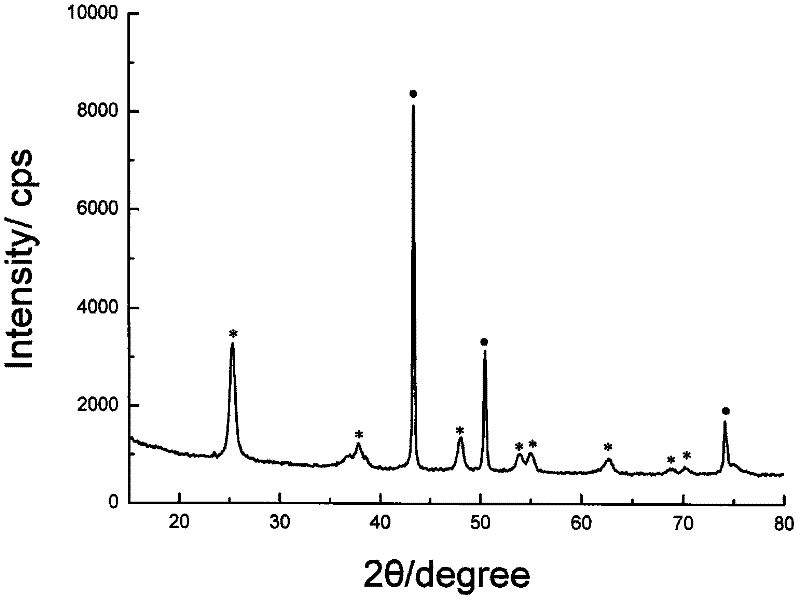
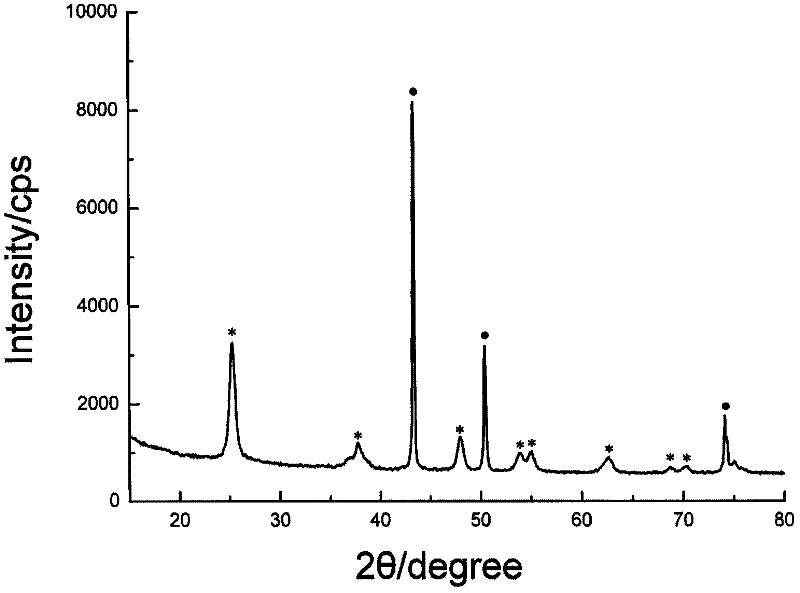
Method for preparing copper-titanium dioxide core-shell nanoparticles
A technology of titanium dioxide and composite nanoparticles, which is applied in the field of preparing copper-titania core-shell nanoparticles, can solve the problems of limited energy utilization in the visible light segment, reduce the efficiency of titanium dioxide photoelectric materials and catalysts, and achieve a mild effect in the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] a. Dissolve cuprous chloride in ammonia solvent, put 5ml cuprous chloride ammonia solution with a concentration of 0.5mol / L in a beaker, and stir for 15min;
[0029] b. Dissolve polyethylene glycol in deionized water, add 5ml of polyethylene glycol 1000 solution with a concentration of 0.005mol / L to step a, and stir for 15 minutes;
[0030] c. Dissolve sodium citrate in deionized water, add 10ml of deionized aqueous solution with a concentration of 0.1mol / L sodium citrate into step b, and stir for 15min;
[0031] d. Dissolve ascorbic acid in deionized water, add 5ml of deionized aqueous solution with a concentration of 0.1mol / L ascorbic acid into step c, and stir for 15min;
[0032] e. Dissolve tetrabutyl titanate in absolute ethanol, add 5 ml of tetrabutyl titanate solution with a concentration of 1.0 mol / L into step d, and stir for 15 minutes;
[0033] f. Add 0.001mol urea to step e, stir for 30min, place the mixed solvent in a 50ml polytetrafluoroethylene liner and ...
Embodiment 2
[0038] a. Dissolve cuprous chloride in ammonia solvent, put 15ml cuprous chloride ammonia solution with a concentration of 0.25mol / L in a beaker, and stir for 15min;
[0039] b. Dissolve polyethylene glycol in deionized water, add 10ml of polyethylene glycol 6000 solution with a concentration of 0.01mol / L to step a, and stir for 15 minutes;
[0040] c. Dissolve sodium citrate in deionized water, add 10ml of deionized aqueous solution with a concentration of 0.5mol / L sodium citrate into step b, and stir for 15min;
[0041] d. Dissolve ascorbic acid in deionized water, add 7ml of deionized aqueous solution of ascorbic acid with a concentration of 0.5mol / L to step c, and stir for 15min;
[0042] e. Dissolve tetrabutyl titanate in absolute ethanol, add 10ml of tetrabutyl titanate ethanol solution with a concentration of 0.5mol / L to step d, and stir for 15 minutes;
[0043] f, add 0.005mol urea in the step e, stir for 30min, place the mixed solvent in the 50ml polytetrafluoroethylen...
Embodiment 3
[0048] a. Dissolve cuprous chloride in ammonia solvent, put 20ml cuprous chloride ammonia solution with a concentration of 0.1mol / L in a beaker, and stir for 15 minutes;
[0049] b. Dissolve polyethylene glycol in deionized water, add 10ml of polyethylene glycol 20000 solution with a concentration of 0.001mol / L to step a, and stir for 15 minutes;
[0050] c. Dissolve sodium citrate in deionized water, add 10ml of sodium citrate solution with a concentration of 1.0mol / L to step b, and stir for 15min;
[0051] d. Dissolve ascorbic acid in deionized water, add 10ml of ascorbic acid solution with a concentration of 1.0mol / L to step c, and stir for 15min;
[0052] e. Dissolve tetrabutyl titanate in absolute ethanol, add 20ml of tetrabutyl titanate ethanol solution with a concentration of 0.1mol / L to step d, and stir for 15 minutes;
[0053] f, add 0.01mol urea in step e, stir for 30min, place the mixed solvent in the stainless steel reaction kettle device of 50ml polytetrafluoroet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com