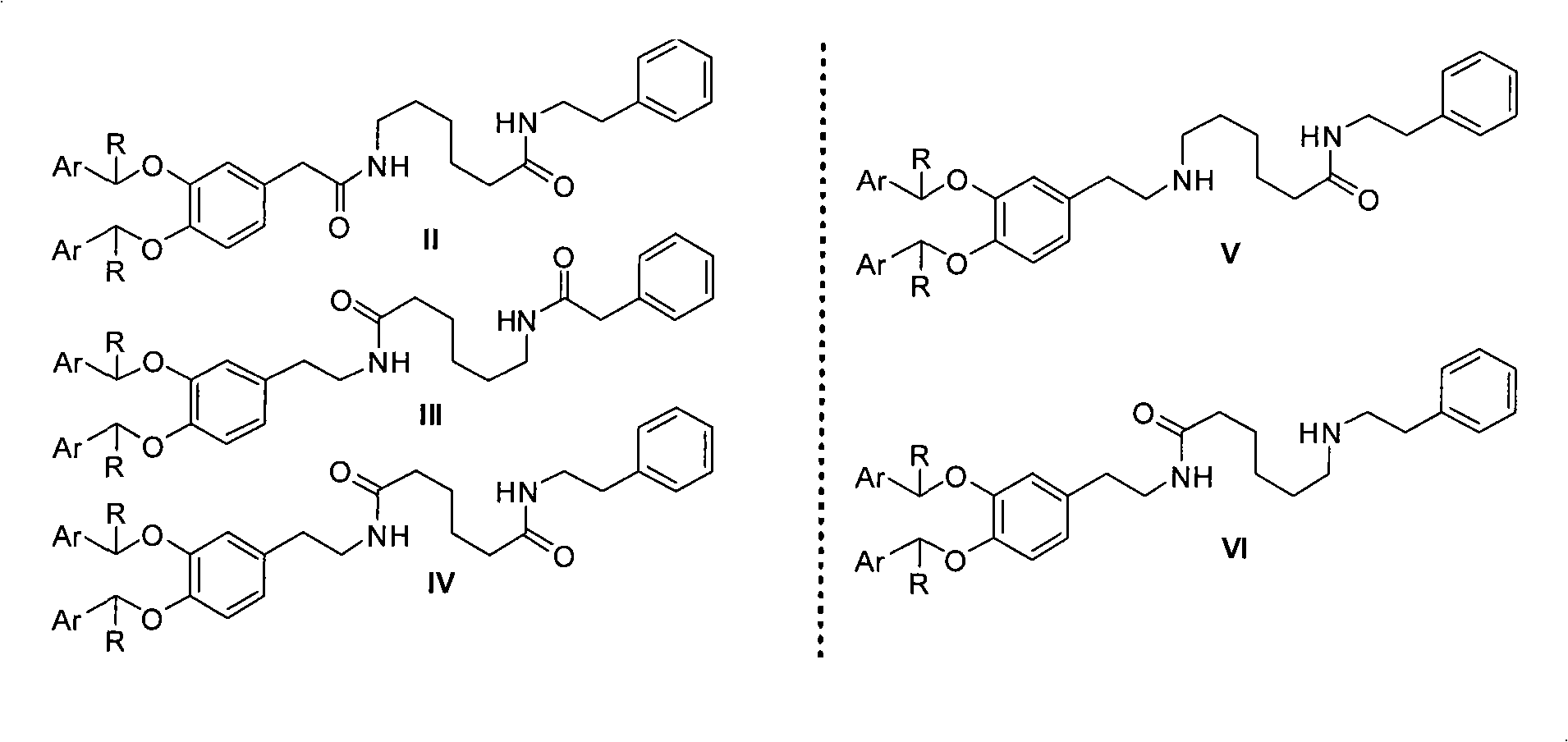
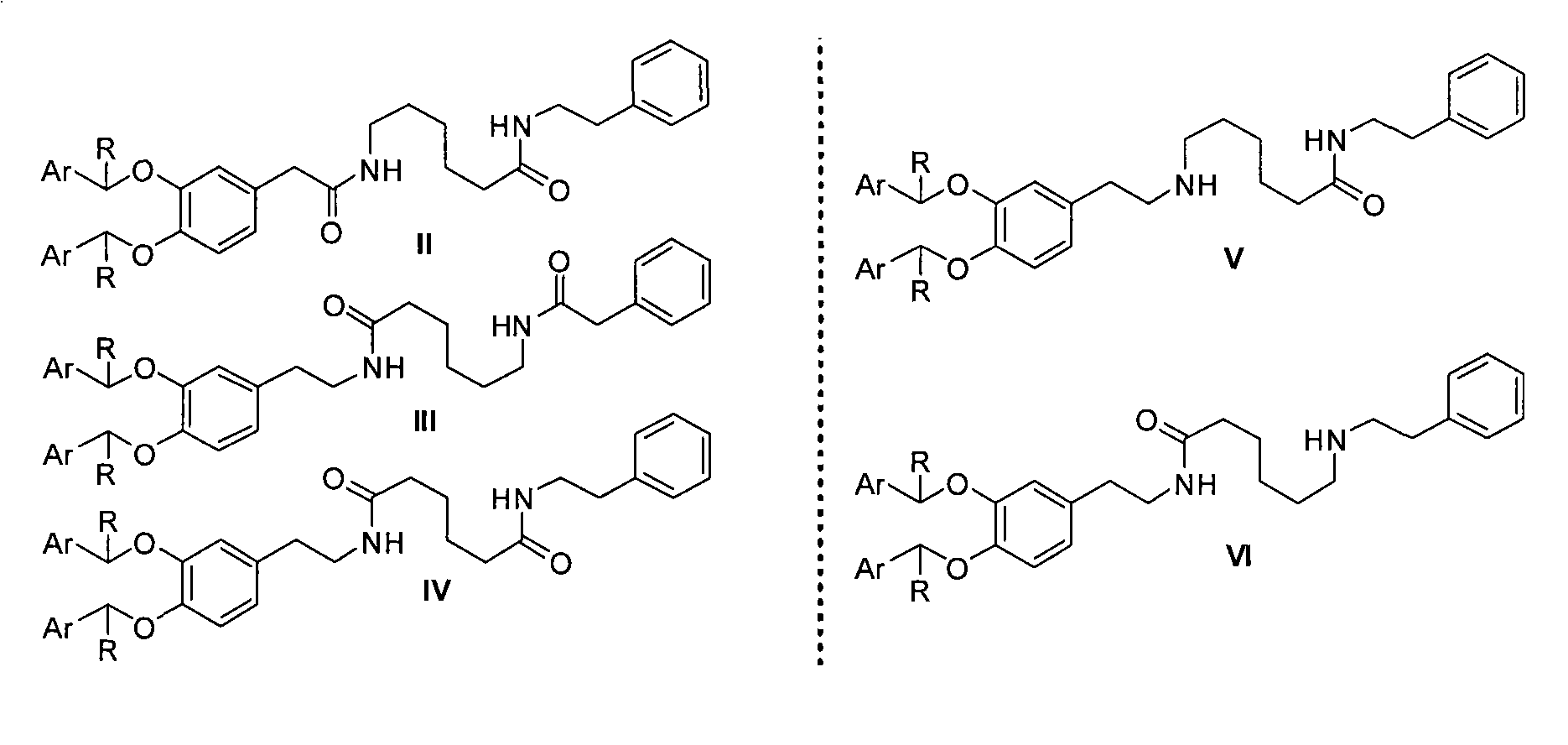
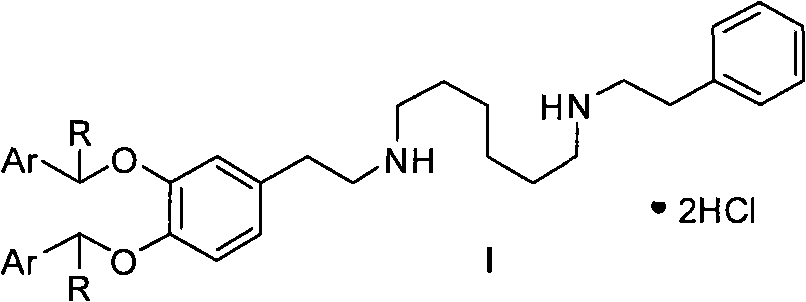
New preparation method of dopexamine hydrochloride by ArCHR protection strategy
A technology of dopexamine hydrochloride and arylmethyl group, which is applied in the field of new preparation of dopexamine hydrochloride, can solve the problems of many by-products, low yield, easy oxidation, etc., and achieve the effect of reducing catalyst poisoning and improving catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]Example 1: Preparation of 6-(2-(3,4-dibenzyloxyphenyl)acetyl)aminocaproic acid (8)
[0023] Below 10°C, the Et 3 N 24.4mL (175mmol) slowly drop to 6-aminocaproic acid 18.3g (140mmol), H 2 O150mL and MeCN 50mL of the mixed solution, stirring while adding. After stirring and dissolving, a solution of 31.2 g (70 mmol) of N-hydroxysuccinimide 3,4-dibenzyloxyphenylacetate in 100 mL of acetonitrile was slowly added dropwise to the mixture. After dropping, the mixture was naturally raised to room temperature, and the stirring was continued for 3 h, and the reaction was basically completed as detected by TLC. Concentrate under reduced pressure to remove most of the solvent, pour the residue into a mixture of 200 mL ethyl acetate and 200 mL ice water, adjust the pH to 2-3 with dilute hydrochloric acid, separate the organic layer, and wash the aqueous layer with ethyl acetate (50 mL×2) After extraction, the combined organic phases were washed with water (100 mL×2), and dried ov...
Embodiment 2
[0025] Example 2: Preparation of 6-(2-(3,4-dibenzyloxyphenyl)acetyl)aminocaproic acid (8)
[0026] Below 10°C, the Et 3 N 24.4mL (175mmol) was slowly dropped into 6-aminocaproic acid 18.3g (140mmol), cyclohexane 150mL and H 2 O 250mL of the mixed solution, stirring while adding. After being dissolved, a solution of 25.7 g (70 mmol) of 3,4-dibenzyloxyphenylacetyl chloride in 100 mL of cyclohexane was slowly added dropwise to the reaction system. After dropping, the mixture was naturally raised to room temperature, and the stirring was continued for 3 h, and the reaction was basically completed as detected by TLC. Adjust the pH to 2-3 with dilute hydrochloric acid, separate the organic layer, extract the aqueous layer with cyclohexane (50mL×2), combine the organic phases, wash with water (100mL×2), and dry over anhydrous sodium sulfate. After filtration, the organic layer was concentrated under reduced pressure to obtain 27.6 g of light yellow solid with a yield of 85.5%.
Embodiment 3
[0027] Example 3: Preparation of 6-(2-(3,4-dibenzyloxyphenyl)acetyl)amino-N-(2-phenylethyl)hexanamide (2)
[0028] At room temperature, add 5.8 g (27.0 mmol) of CDI to 6-(2-(3,4-dibenzyloxyphenyl) acetyl) aminocaproic acid (8) 8.3 g (18.0 mmol) of CH 2 Cl 2 After stirring for 2 hours in 200 mL of the solution, 4.5 mL (27.0 mmol) of 2-phenylethylamine was added, and the stirring was continued for 5 hours. TLC detected that the reaction was basically completed. The reaction solution was poured into 300 mL of ice water, the organic layer was separated, and the aqueous layer was washed with CH 2 Cl 2 (50mL×2) for extraction, combined the organic phases, washed with water (100mL×2), and dried over anhydrous sodium sulfate. After filtration and concentration, 8.8 g of white solid was obtained with a yield of 86.3%.
[0029] MS (m / z): [M+H] + 565.3, [M+Na] + 587.3, [M-H] - 563.1, [M+Cl] - 599.1.
[0030] 1 H NMR (CDCl 3 , 600MHz) δ=1.19~1.21(2H, m), 1.36~1.38(2H, m), 1.54...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com