Process for producing lithium borate compound
A compound, lithium borate technology, applied in the direction of boron compounds, boron oxide compounds, chemical instruments and methods, etc., can solve the problems of slow ion diffusion, lithium-deficient borate compounds, high resistance, etc., to achieve high capacity and good cycle characteristics, effect of inhibiting grain growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Synthesis of lithium-excess borate-based compounds and charge-discharge characteristics of batteries using the compounds
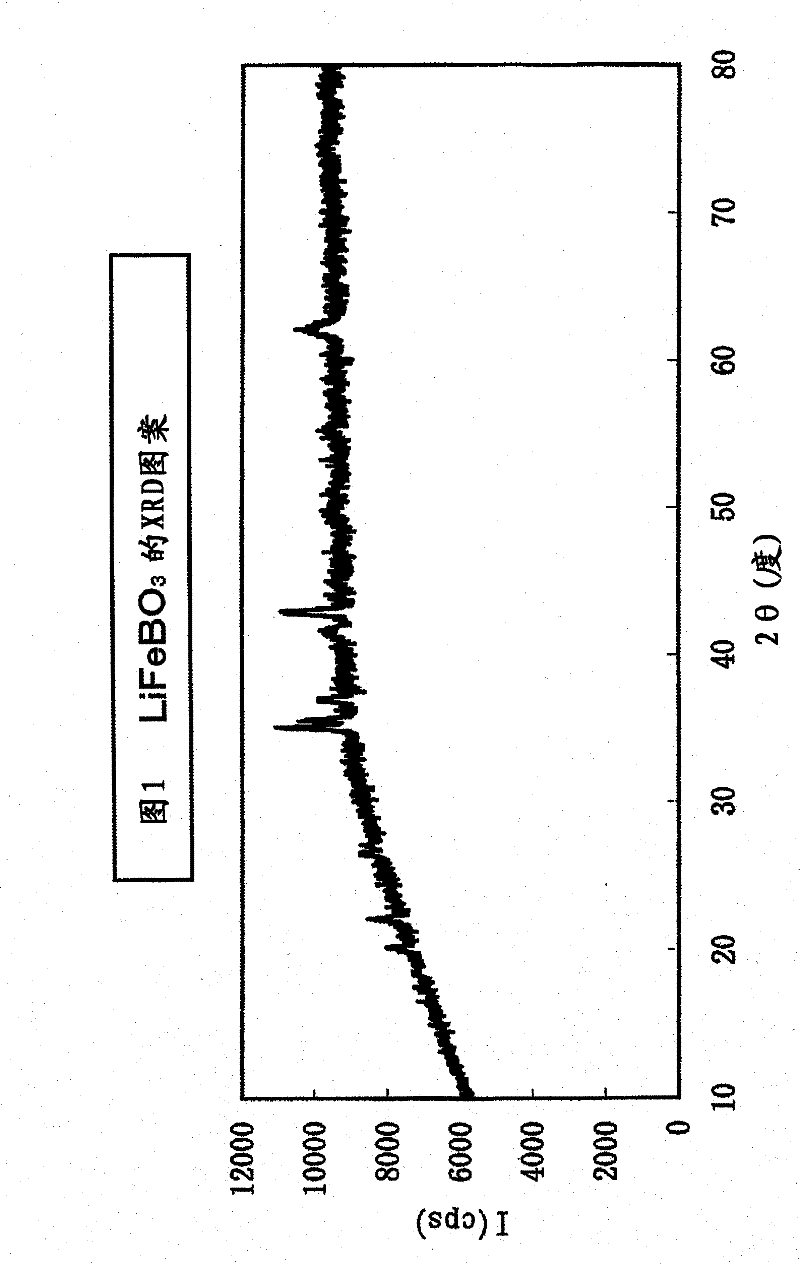
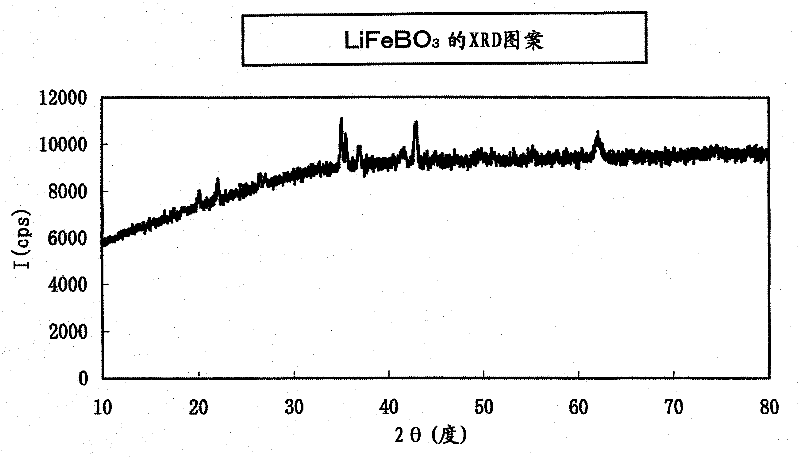
[0080] As a raw material, iron oxalate FeC is used 2 o 4 2H 2 O (manufactured by SIGMA-ALDRICH, purity 99.99%), lithium hydroxide (anhydrous) LiOH (KISHIDA chemical, 98%), boric acid H 3 BO 3 (KISHIDA Chemical, 99.5%) each 0.005 mole, they are mixed with carbonate mixture (lithium carbonate (KISHIDA Chemical, passivation 99.9%), sodium carbonate (KISHIDA Chemical, purity 99.5%) and potassium carbonate (KISHIDA Chemical system, purity 99.5%)) mixed at a molar ratio of 0.435:0.315:0.25) were mixed. The mixing ratio is such that the total amount of iron oxalate, lithium hydroxide, and boric acid is 225 parts by weight relative to 100 parts by weight of the carbonate mixture.
[0081] 20 mL of acetone was added thereto, mixed with a zirconia ball mill at 500 rpm for 60 minutes, and dried. Then, the obtained powder was heated in a gold crucible, a...
Embodiment 2
[0095]Use the ferric oxalate used in the method of embodiment 1 and the metal component corresponding to the target composition shown in following table 2, except that, carry out the operation identical with embodiment 1 and synthesize by composition formula: Li 1+a-b A b m 1-x M' x BO 3+c (In the formula, A is at least one element selected from Na, K, Rb and Cs, M is at least one element selected from Fe and Mn, M' is selected from Mg, Ca, Co, Al, Ni , Nb, Mo, W, Ti and Zr. Each subscript is as follows: 0≤x≤0.5, 0 b) Lithium-excess borate-based compound represented. In addition, instead of the iron oxalate used in the method of Example 1, a metal component corresponding to the target composition shown in Table 3 below was used, and the same operation as in Example 1 was performed to synthesize the compound represented by the above composition formula. A lithium-excess borate-based compound.
[0096] It should be noted that the use of iron oxalate FeC 2 o 4 2H 2 O (ma...
Embodiment 3
[0112]
[0113] Add 50 parts by weight of acetylene black (hereinafter referred to as AB) and 20 parts by weight of LiF to 100 parts by weight of the product (lithium borate-based compound) after removing water-soluble substances such as carbonates in Example 2, and use a planetary ball mill (5mm Zirconia balls) were ground at 450rpm for 5 hours, in the mixed gas of carbon dioxide and hydrogen ((CO 2 :H 2 Heat treatment was performed at 700° C. for 2 hours in an atmosphere of (molar ratio)=100:3). The XRD pattern of the product after the heat treatment was very consistent with that of the sample before the heat treatment, and it was confirmed that the lithium-excess borate-based compound was not decomposed and the crystal structure was maintained. In addition, the elemental analysis results (element molar ratios) obtained by the ICP method are shown in Tables 6 and 7 below. From these tables, it was confirmed that the products were all lithium-excess fluorine-containing li...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com