Synthesis method of hydroquinone
A technology of hydroquinone and synthesis method, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry and other directions, can solve the problems of difficult catalyst recovery, low molar ratio, high toxicity, etc. Low product, mild effects of temperature and pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
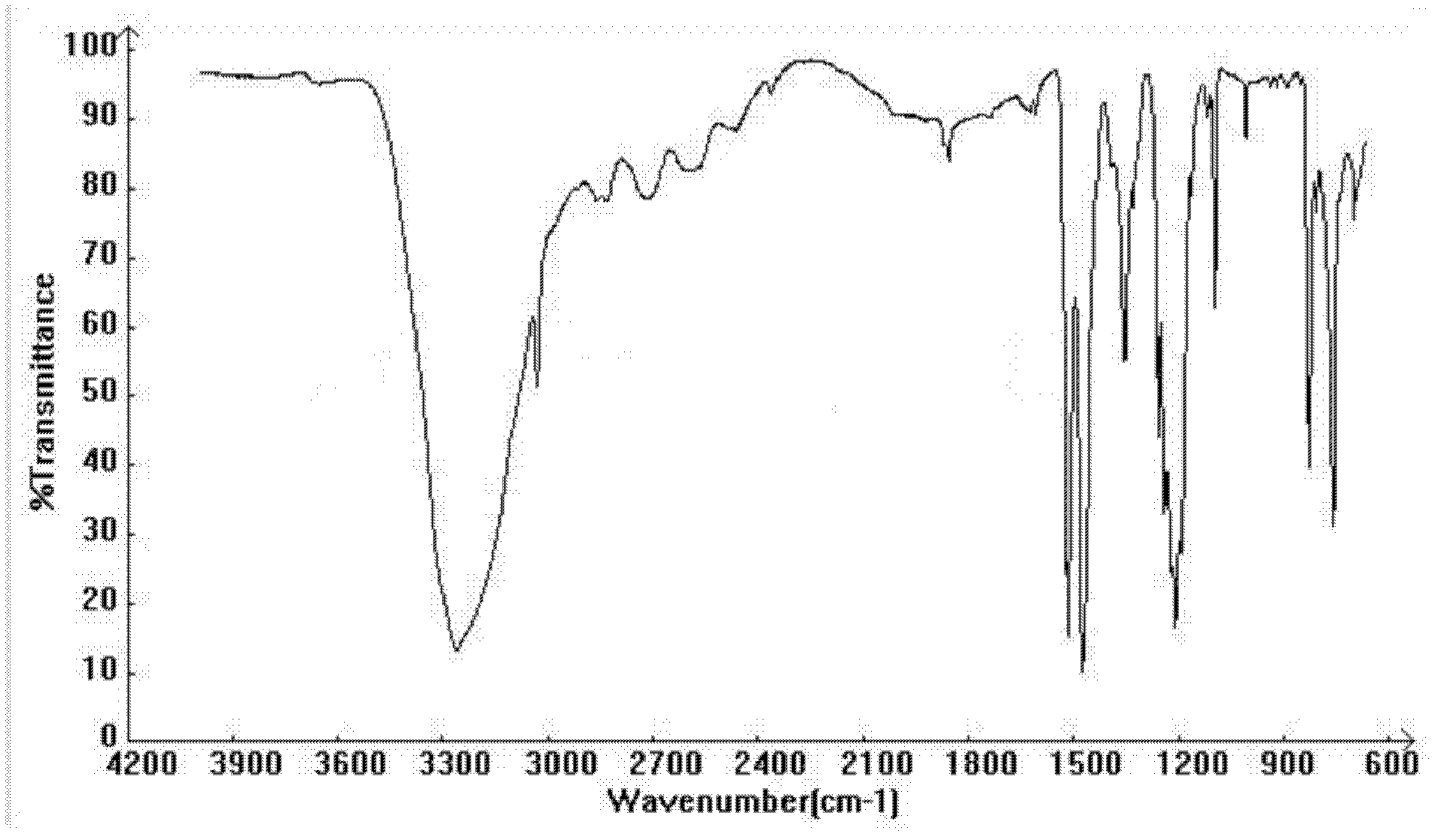
Image
Examples
Embodiment 1
[0029] (1) Take 10 g phenol and 5 g copper sodium compound salt (CuSO 4 : NaCl = 1:3, purchased from Shanghai Reagent Factory) was added into a three-necked flask containing 50 mL of methanol, fed with oxygen, refluxed at 60°C for 6 hours, and monitored by TLC.
[0030] (2) Distill methanol off, add 10 mL of water to the residue, and steam distill until the distillate is colorless. Concentrate, cool, filter, and isolate 8.62 g of p-benzoquinone from the distillate (yield: 75.0 %). The mother liquor was concentrated by steam distillation, cooled, and filtered to isolate 2.07 g of phthaloquinone (yield: 18.0 %).
[0031] (3) Take 8.62 g of p-quinone and 9.0 g of ammonium thiosulfate / ammonium sulfate mixture (ammonium thiosulfate 68.5wt%, ammonium sulfate 31.5wt%, the residue after extracting potassium thiocyanate from Nangang steelmaking wastewater) Add it into 17 mL of hot water, stir for 20 minutes, adjust the pH value to 1 with sulfuric acid, cool, and precipitate the crude...
Embodiment 2
[0035] (1) Take 50 g phenol and 5 g copper sodium compound salt (CuSO 4 :NaCl=1:2, CuSO 4 and NaCl were purchased from Shanghai Reagent Factory) into a three-necked flask containing 250 mL of ethanol, fed with oxygen, refluxed at 70°C for 2 hours, and monitored the reaction progress by TLC.
[0036] (2) Ethanol was distilled off, 50 mL of water was added to the residue, and steam distilled until the distillate was colorless. Concentrate, cool, filter, and isolate 43.3 g of p-benzoquinone from the distillate (yield: 75.4%). The mother liquor was concentrated by steam distillation, cooled, and filtered to isolate 10.2 g of phthaloquinone (yield: 17.8 %).
[0037] (3) Take 43.3 g of p-quinone and 45.0 g of ammonium thiosulfate / ammonium sulfate mixture (ammonium thiosulfate 68.5wt%, ammonium sulfate 31.5wt%, the residue after extracting potassium thiocyanate from Nangang steelmaking wastewater) Add it into 85 mL of hot water, stir for 20 minutes, adjust the pH value to 1 with s...
Embodiment 3
[0041] (1) Take 100 g phenol and 7.5 g copper-sodium compound salt (Cu(NO 3 ) 2 : NaCl = 1:3, Cu(NO 3 ) 2 and NaCl (purchased from Shanghai Reagent Factory) were added to a three-necked flask containing 500 mL of propanol, heated (at a temperature of about 70°C), fed with oxygen, and the reaction was monitored by TLC, and the reaction was complete within 2 hours.
[0042] (2) Ethanol was distilled off, 100 mL of water was added to the residue, and steam distilled until the distillate was colorless. Concentrate, cool, filter, and isolate 87.2 g of p-benzoquinone from the distillate (yield: 75.9 %). The mother liquor was concentrated by steam distillation, cooled and filtered to isolate 20.1 g of phthaloquinone (yield: 17.5 %).
[0043] (3) Take 87.2 g of p-quinone and 90.7 g of ammonium thiosulfate / ammonium sulfate mixture (ammonium thiosulfate 68.5wt%, ammonium sulfate 31.5wt%, the residue after extracting potassium thiocyanate from Nangang steelmaking wastewater) Add it ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com