Bendamustine hydrochloride crystal and preparation method thereof
A technology of bendamustine hydrochloride and bendamustine hydrochloride is applied in the field of polymorphic form of bendamustine hydrochloride, and can solve the problems of complicated operation, unsuitable for industrialized production, and difficulty of solvent meeting pharmacopoeia standards, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Preparation of bendamustine hydrochloride polymorph I
[0059] Take 10 grams of bendamustine hydrochloride crude product and place it in a reaction flask, add 50ml of 1mol / L hydrochloric acid solution, heat to 70-80°C, dissolve, slowly cool to room temperature and stir for 1 hour, then cool down to 0-10°C to analyze Crystal for 5 hours. Filter, wash the filter cake with water, and wash with acetone.
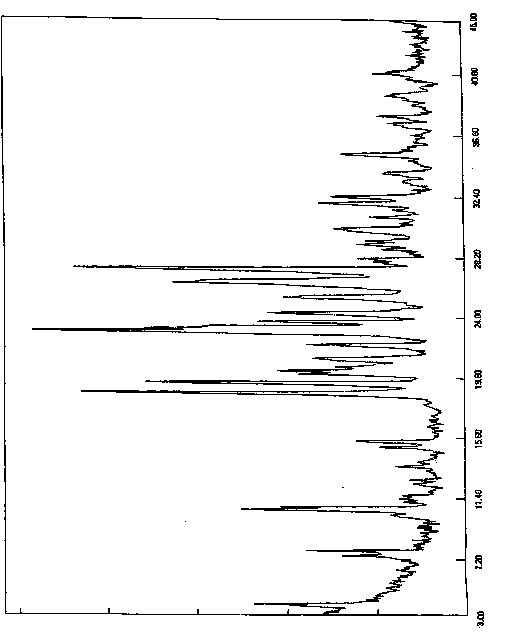
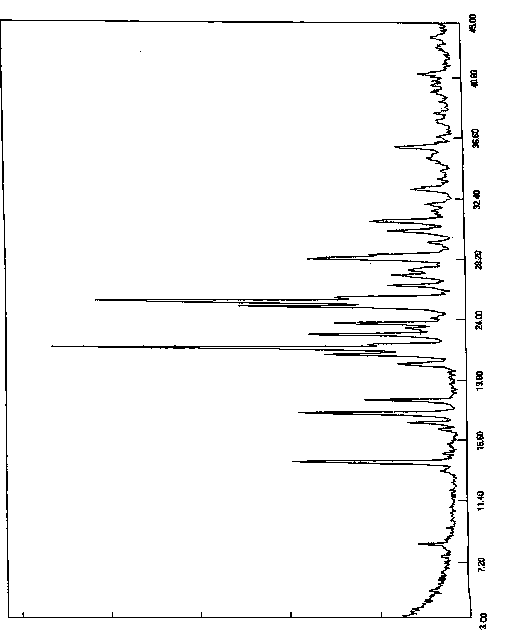
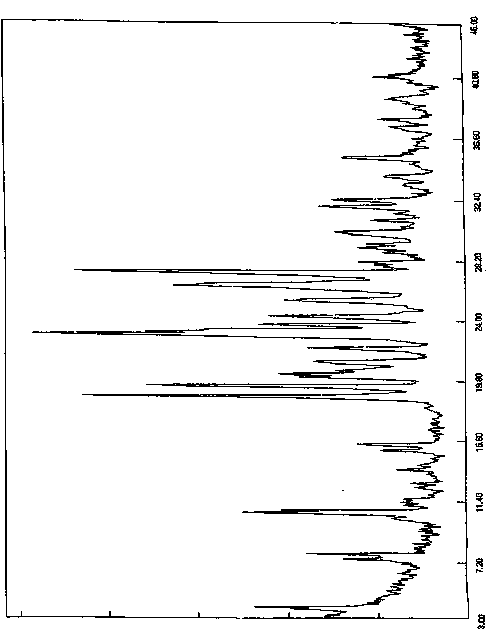
[0060] The resulting solid was added to a reaction flask, 300ml of acetone was added, heated to reflux and stirred for 2 hours, slowly cooled to room temperature and stirred for 1 hour, then cooled to 0-10°C for 5 hours of crystallization. Filter, wash the filter cake with acetone, collect the solid, and dry under reduced pressure (-0.09MPa) at 45°C with phosphorus pentoxide as an aid. Obtained 8.2 grams of white solid, yield 82%, purity 99.92%, X-diffraction data are the same as Table 1.
Embodiment 2
[0061] Example 2 Preparation of bendamustine hydrochloride polymorph I
[0062] Take 10 grams of bendamustine hydrochloride crude product and place it in a reaction flask, add 40ml of 1mol / L hydrochloric acid solution, heat to 70-80°C, dissolve, slowly cool to room temperature and stir for 1 hour, then cool down to 0-10°C to analyze Crystal for 5 hours. Filter, wash the filter cake with water, and wash with acetone.
[0063] The resulting solid was added to a reaction flask, 400ml of acetone was added, heated to reflux and stirred for 2 hours, slowly cooled to room temperature and stirred for 1 hour, then cooled to 0-10°C for 5 hours of crystallization. Filter, wash the filter cake with acetone, collect the solid, and dry under reduced pressure (-0.09MPa) at 45°C with phosphorus pentoxide as an aid. Obtained 8.4 grams of white solid, yield 84%, purity 99.90%, X-diffraction data are the same as Table 1.
Embodiment 3
[0064] Example 3 Preparation of bendamustine hydrochloride polymorph I
[0065] Take 7.6 grams of bendamustine hydrochloride crude product and place it in a reaction flask, add 45ml of 1mol / L hydrochloric acid solution, heat to 70-80°C, dissolve, slowly cool to room temperature and stir for 2 hours, then cool down to 0-10°C to analyze Crystal 6 hours. Filter, wash the filter cake with water, and wash with acetone.
[0066] The resulting solid was added to a reaction flask, 270ml of acetone was added, heated to reflux and stirred for 1.5 hours, slowly cooled to room temperature and stirred for 2 hours, then cooled to 0-10°C for 8 hours of crystallization. Filter, wash the filter cake with acetone, collect the solid, and dry under reduced pressure (-0.09MPa) at 45°C with phosphorus pentoxide as an aid. 8.0 g of white solid was obtained, with a yield of 80% and a purity of 99.96%. The X-diffraction data are the same as in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com