Method for manufacturing olefin
A manufacturing method and technology of olefins, applied in the field of olefins manufacturing, capable of solving problems such as performance degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0197] Hereinafter, the present invention will be described in more detail based on examples, but the present invention is not limited thereto.
[0198] [Catalyst preparation]
[0199] (Preparation Example 1-1) Preparation of Metathesis Catalyst WQ-15
[0200] In SiO 2 After the carrier (Fuji Silysia, CARiACT Q-15) was impregnated with ammonium metatungstate, it was fired at 500°C in an air atmosphere to obtain WO 3 / SiO 2 catalyst. The catalyst obtained by pulverizing and classifying the catalyst into a size of 150 to 500 μm was designated as WQ-15.
preparation example 1-2
[0201] (Preparation Example 1-2) Preparation 1 of an isomerization catalyst containing Mg and Y
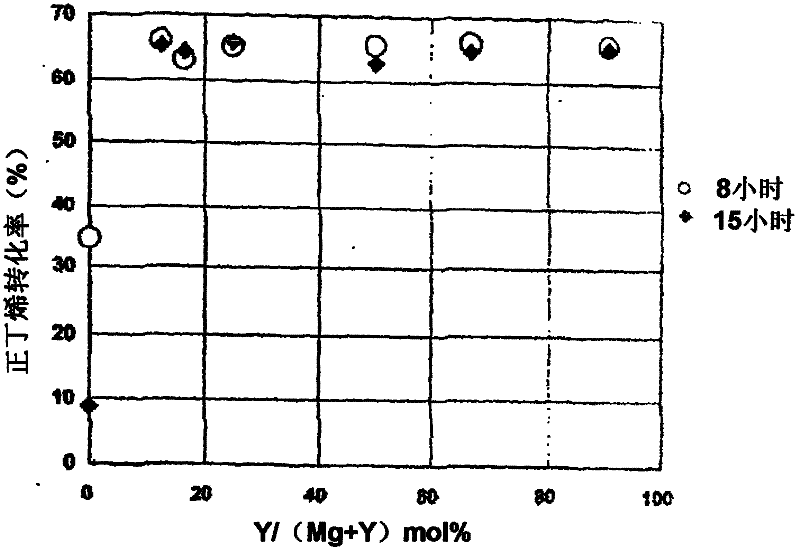
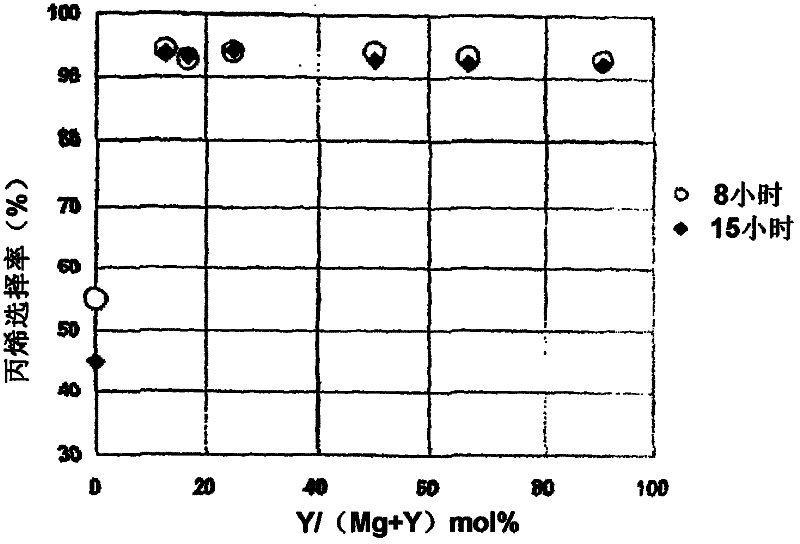
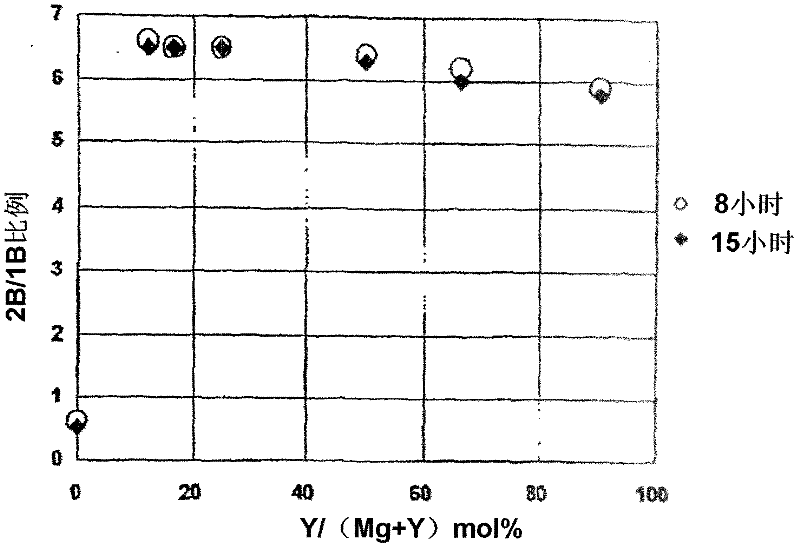
[0202] The isomerization catalyst containing Mg and Y is prepared by changing the molar ratio of the metal nitrate used to prepare a catalyst with Mg / Y molar ratio = 0.1, 0.5, 1, 3, 5 or 7.
[0203] A mixed aqueous solution of magnesium nitrate and yttrium nitrate and 25% aqueous ammonia were prepared, and the solution was sent to a flask filled with water and mixed. At this time, the liquid delivery speed of ammonia water was controlled so that the pH of the liquid in the flask was maintained at 10. After mixing, add appropriate amount of ammonia water until the pH of the liquid in the flask does not change any more, and perform aging. The resulting precipitate was repeatedly filtered and redispersed in water to be washed. After drying the obtained wet cake, it fired at 550 degreeC in air atmosphere, and obtained the metal oxide containing Mg and Y. It was made into powder, th...
preparation example 1-3
[0205] (Preparation Example 1-3) Preparation 2 of an isomerization catalyst containing Mg and Y
[0206] A mixed aqueous solution of magnesium nitrate and yttrium nitrate and an aqueous sodium hydroxide solution were prepared. Next, water in which carbonate ions were dissolved in sodium carbonate in an equimolar amount with respect to the yttrium salt was prepared in the flask, and the nitrate aqueous solution and the sodium hydroxide aqueous solution were mixed while feeding the solution while keeping the temperature at 60°C. At this time, the liquid feeding rate of the sodium hydroxide aqueous solution was controlled so that the pH of the liquid in the flask was maintained at 10. After the mixing was completed, heating was further performed, and aging was carried out under reflux for 2 hours. The resulting precipitate was repeatedly filtered and redispersed in water to be washed. After drying the obtained wet cake, it fired at 550 degreeC in air atmosphere, and obtained th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com