Magnetic resonance contrast agent constructed by amphipathic polysaccharide-wrapped super-paramagnetic nanoparticles and preparation method thereof
A technology of magnetic resonance contrast agent and amphiphilic polysaccharide, which is applied in the directions of preparations, pharmaceutical formulations, and emulsion delivery for in vivo experiments to achieve the effects of high grafting degree, simple operation and good magnetic properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0056] The amphiphilic dextran (Dex T10 -g-SA) Preparation
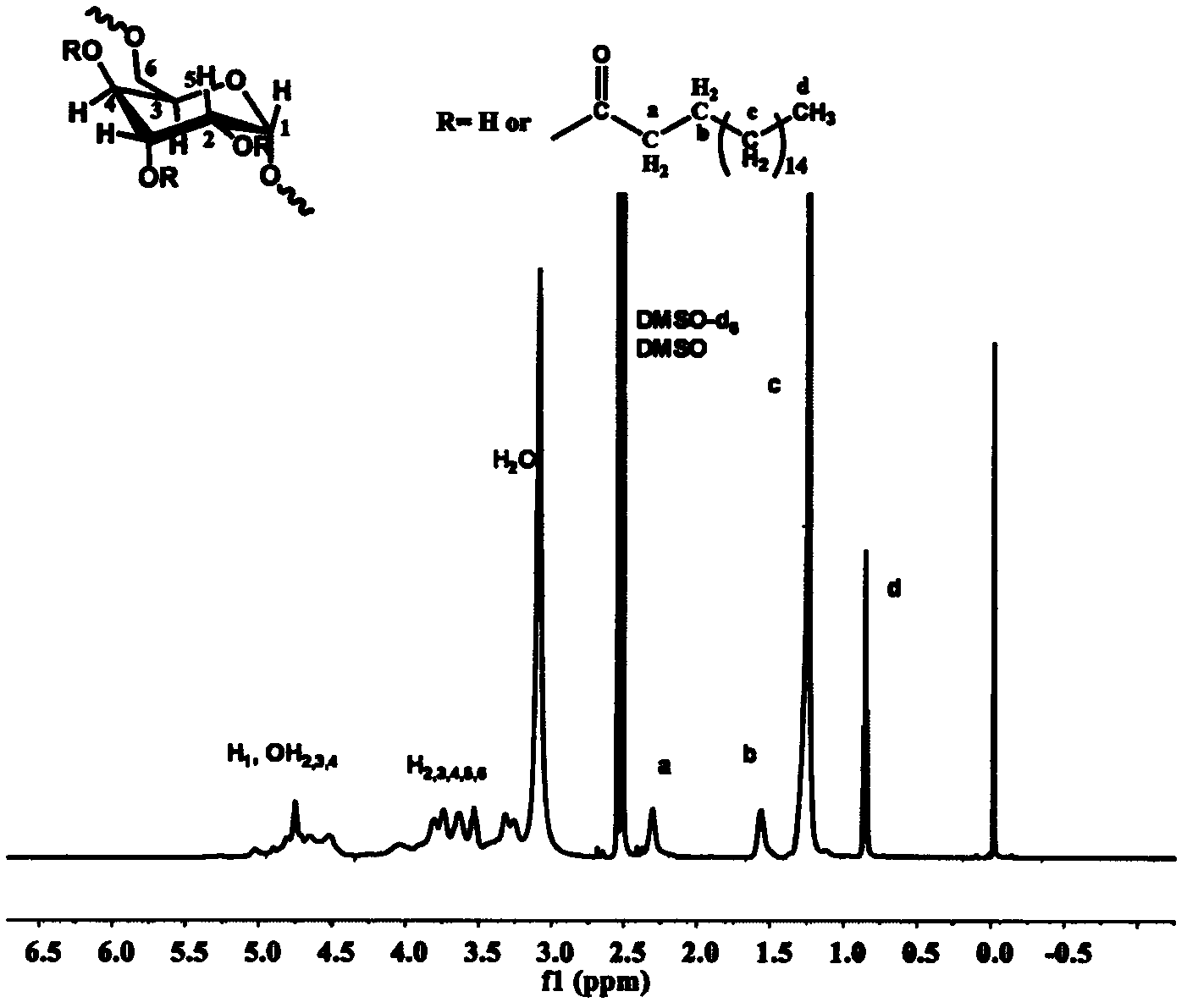
[0057] Weigh 0.284g (1mmol) of stearic acid and 0.171g of N,N'-carbonyldiimidazole (1.05mmol) and dissolve it in 4mL of dry tetrahydrofuran. Under the protection of argon, reflux at a temperature of 80°C for 3h to obtain the solution ① for later use; Weigh 0.202 g of dextran with a molecular weight of 10,000 g / mol and dissolve it in 10 mL of dry dimethyl sulfoxide, heat until completely dissolved, inject solution ① into the reaction solution under the protection of argon, and heat the obtained solution to 130 ° C for 5 h , the solution obtained by the reaction was added to 300 mL ethyl acetate to precipitate and wash three times, and centrifuged to obtain a white solid product. product of 1 H NMR spectrum (DMSO-d 6 / CDCl 3 v:v=1 / 1) Proton hydrogen chemical shift is assigned as figure 1 shown. The results showed that stearic acid was successfully grafted onto dextran, from 1 The H NMR spectrogram can calculate ...
example 2
[0058] The amphiphilic dextran (Dex) of example 2 stearic acid modification T40 -g-SA) Preparation
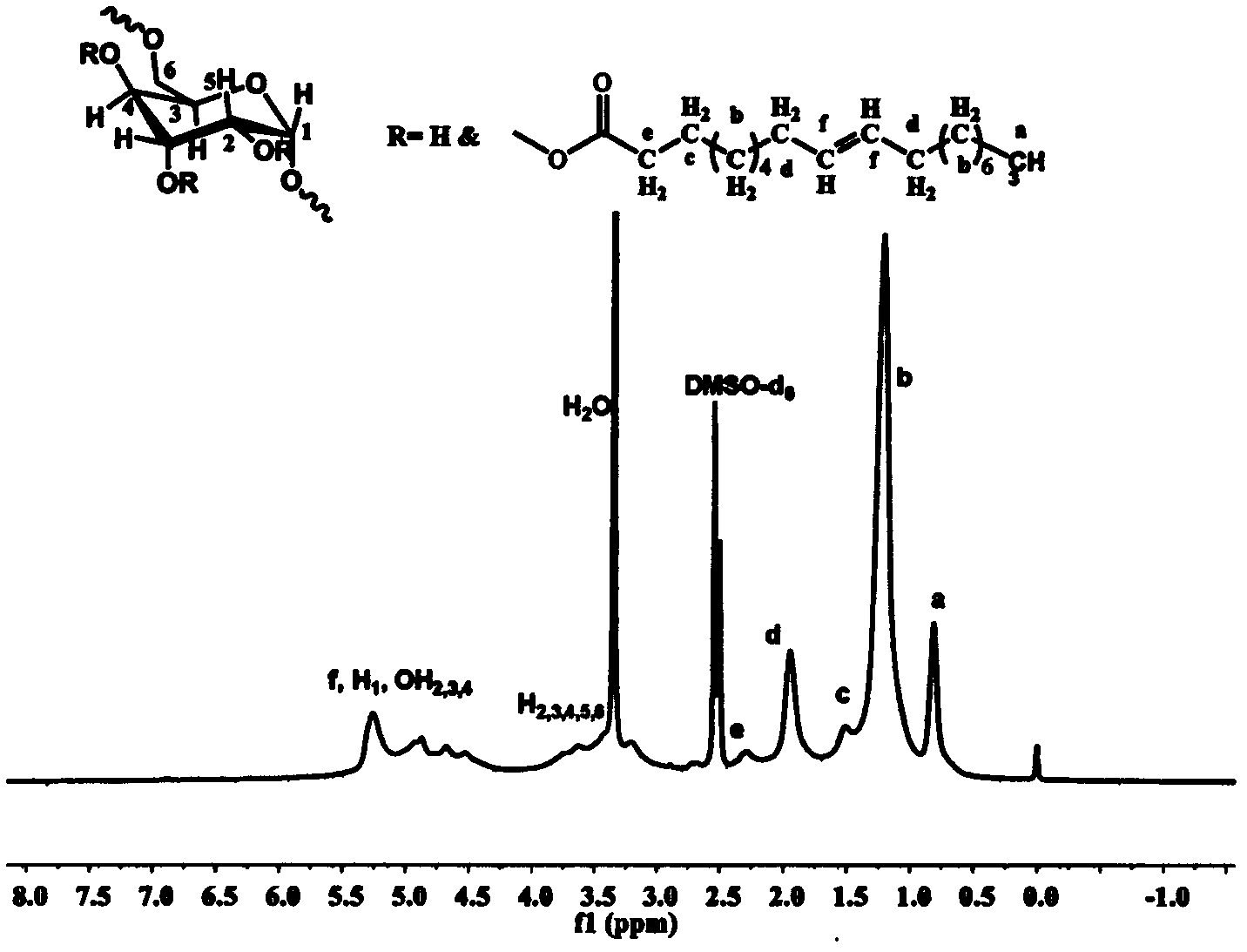
[0059] Weigh 0.426g (1.5mmol) of stearic acid and 0.255g of N,N'-carbonyldiimidazole (1.58mmol) and dissolve them in 6mL of dry tetrahydrofuran. Under the protection of argon, reflux at a temperature of 80°C for 3h to obtain a solution ② for later use Weigh 0.243g of dextran with a molecular weight of 40000g / mol and dissolve it in 15mL of dry dimethyl sulfoxide, heat until completely dissolved, inject solution ② into the reaction solution under the protection of argon, and heat the obtained solution to 130°C for reaction After 5 h, the solution obtained from the reaction was added to 300 mL of ethyl acetate to precipitate and wash three times, and centrifuged to obtain a white solid product.
example 3
[0060] The amphiphilic dextran (Dex) of example 3 stearic acid modification T70 -g-SA) Preparation
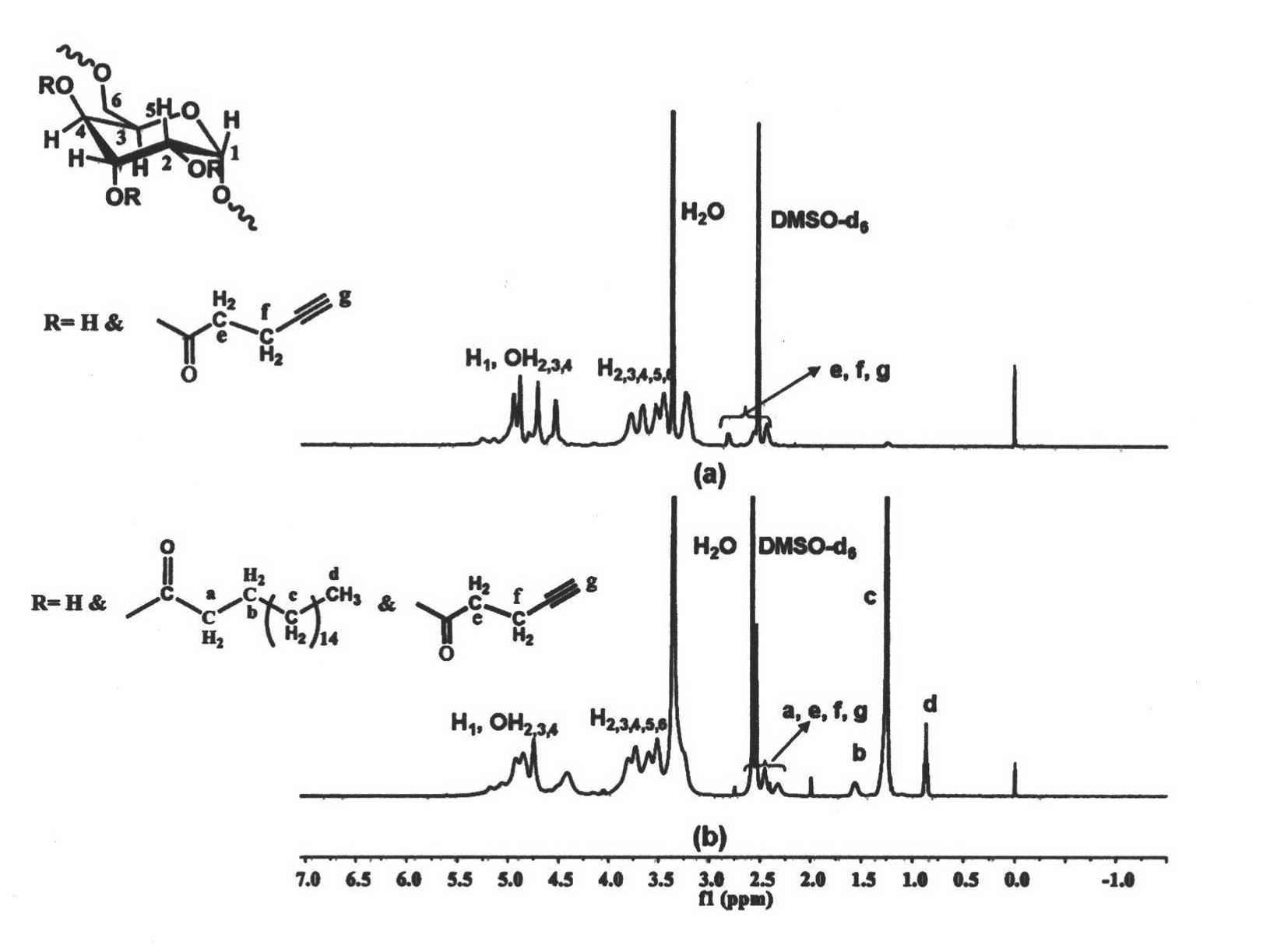
[0061] Weigh 0.426g (1.5mmol) of stearic acid and 0.267g of N,N'-carbonyldiimidazole (1.65mmol) and dissolve them in 6mL of dry tetrahydrofuran. Under the protection of argon, reflux at a temperature of 80°C for 3h to obtain a solution ③ for later use Weigh 0.270 g of dextran with a molecular weight of 70,000 g / mol and dissolve it in 15 mL of dry dimethyl sulfoxide, heat until completely dissolved, inject solution ③ into the reaction solution under the protection of argon, and heat the obtained solution to 130°C for reaction After 5 h, the solution obtained from the reaction was added to 300 mL of ethyl acetate to precipitate and wash three times, and centrifuged to obtain a white solid product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com