Gas purifying method and gas purifying apparatus
A technology for gas purification and raw gas, applied in chemical instruments and methods, inert gas compounds, separation methods, etc., can solve the problems of high manufacturing costs and expensive adsorbents, and achieve a reduction in filling amount, filling amount, and manufacturing. cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
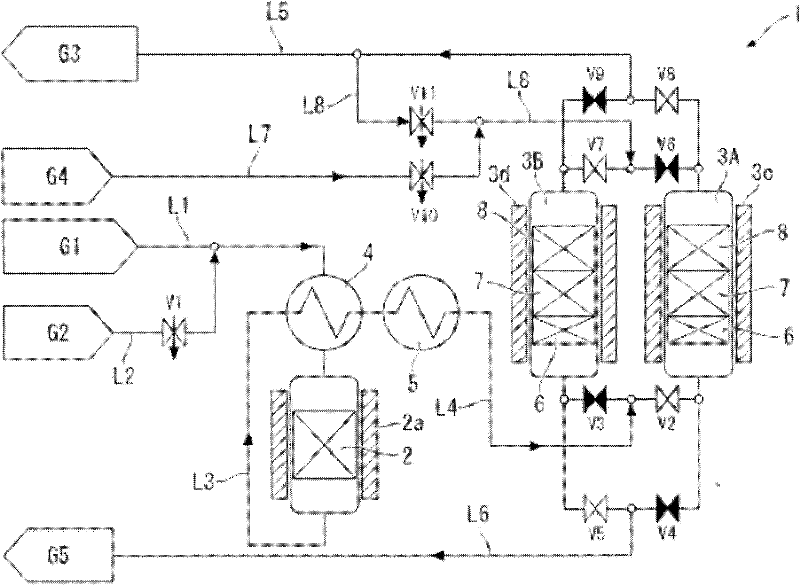
[0096] A stainless steel cylinder with an inner diameter of 100 mm was filled with a 400 mm alumina-supported Pd catalyst as the catalyst tower 2 . A moisture adsorbent layer 6 (MS5A) consisting of a zeolite layer with a thickness of 100 mm, a nickel catalyst layer 7 (N112) with a thickness of 50 mm, and a 250 mm-thick The alumina layer 8 containing sodium in a weight ratio of 5.8% was used as the adsorption tower 3A.
[0097] First, each layer of the adsorption tower 3A was regenerated under the following conditions.
[0098] First, make nitrogen containing hydrogen concentration 2vol% pass through passage L7 and passage L8 at 2Nm 3 The flow rate per hour was introduced into the adsorption tower 3A for 3 hours, and heated to 200°C by the heater 3c. Next, in nitrogen at 2Nm 3 After the flow rate per hour flows into the adsorption tower 3A, the adsorption tower 3A is cooled.
[0099] Next, an adsorption step is performed.
Embodiment 2
[0103] A stainless steel cylinder with an inner diameter of 100 mm was filled with a 400 mm alumina-supported Pt catalyst as the catalyst tower 2 . A moisture adsorbent layer 6 (MS5A) consisting of a zeolite layer with a thickness of 100 mm, a nickel catalyst layer 7 (N112) with a thickness of 50 mm, and a thickness of A 250mm alumina layer 8 containing sodium in a weight ratio of 5.8% was used as the adsorption tower 3A.
[0104] Next, after regenerating the adsorption tower 3A under the same conditions as in Example 1, an adsorption step was performed under the same conditions as in Example 1.
[0105] Oxygen was detected as the first breakthrough component 49 hours after the start of introduction of the raw material gas into the catalyst tower 2 and the adsorption tower 3A.
Embodiment 3
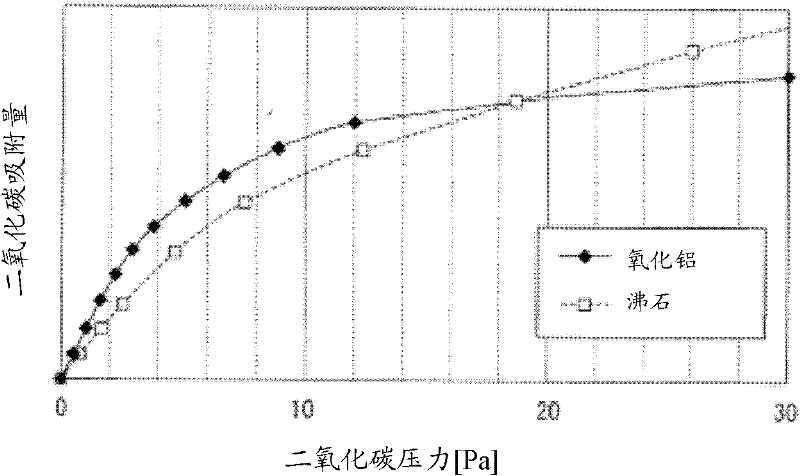
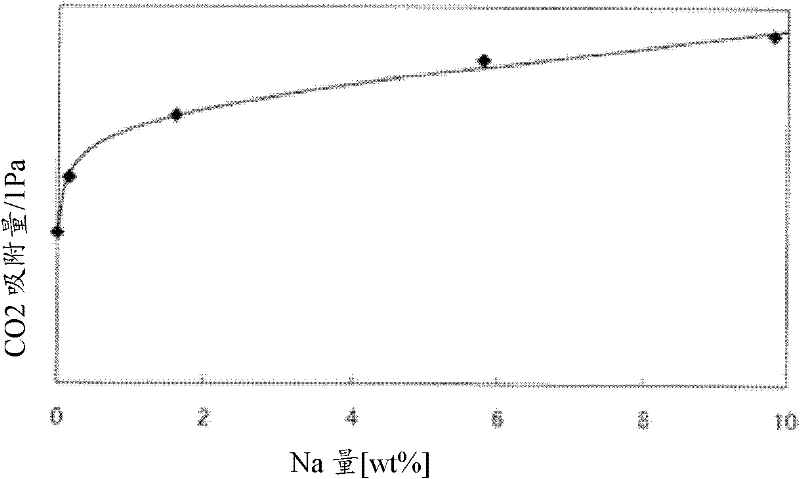
[0124] Under the condition that the partial pressure of carbon dioxide is 1 Pa, the carbon dioxide adsorption capacity of alumina is determined by changing the sodium content. As a result, compared with the carbon dioxide adsorption capacity of alumina with a sodium content of 0.1wt%, 1.6wt%, 5.8wt%, and 9.8wt%, which were 38, 50, 60, and 65 mmol / kg, respectively, alumina with a sodium content of 0.1wt% or less The carbon dioxide adsorption capacity is 28mmol / kg. The results are shown in the graph where the horizontal axis is the sodium content and the vertical axis is the carbon dioxide adsorption amount. image 3 middle. image 3 The carbon dioxide adsorption amount shown was measured using a constant volume type gas adsorption amount measuring device (Baratron electrostatic volumetric manometer (manufactured by MKS Co., Ltd.) with a full scale of 13.2 Pa) at a set temperature of 25° C. and a pressure of 1Pa to carry out.
[0125] Depend on image 3 It can be seen from t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com