Method for synthesizing panaxytriol
A technology of panaxatriol and diol, which is applied in chemical instruments and methods, preparation of oxygen-containing compounds, preparation of hydroxyl compounds, etc., and can solve the problems of long synthesis route and low synthesis yield of panaxatriol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
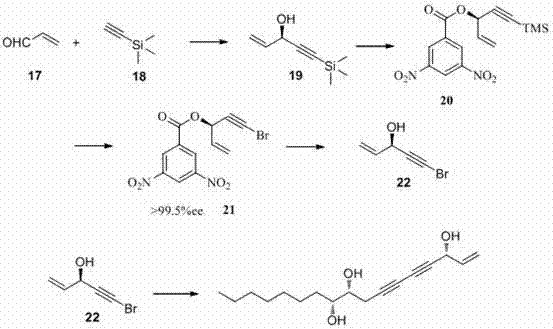
[0054] preparation Panaxytriol (I)
[0055] 0.0118g (0.12 mmol) cuprous chloride, 0.16mL n-butylamine, 0.37mL water, 0.118 g (0.6mmol) (4 R ,5 R )-dodecane-1-yne-4,5-diol in dichloromethane (9mL) solution was slowly added 0.144g (0.9mmol) ( R )-5-bromo-1-penten-4-yn-3-ol, 0.2081g (3mmol) hydroxylamine hydrochloride aqueous solution 2mL, keep the system light yellow, after the dropwise addition, continue to react for 1h. After the reaction, extract with dichloromethane, combine the organic phases, dry over anhydrous sodium sulfate, filter, concentrate, and perform silica gel column chromatography (petroleum ether: ether, 5:1) to obtain 0.14 g of light yellow oily liquid (yield: 85 %). [α] D 20 = -20.8 (c = 1.1, CHCl 3 ); 1 H NMR (300 MHz, CDCl 3 ): δ 5.95 (ddd, J = 17.04, 10.11, 5.31 Hz 1H), 5.44-5.50 (m, 1H), 5.23-5.27 (m, 1H), 4.90-4.94 (m, 1H), 3.62 (m, 2H ), 2.58 (m, 2H), 2.11 (br 3H), 1.25–1.58 (m, 12H), 0.88 (t, J = 6.87 Hz 3H); 13 C NMR (75 MHz, CDCl 3 ): Δ...
Embodiment 2
[0057] preparation Panaxytriol ( Ⅰ )
[0058] 0.0177g (0.12 mmol) cuprous bromide, 0.16mL ethylamine, 0.37mL water, 0.118g (0.6mmol) (4 R ,5 R )-dodecane-1-yne-4,5-diol in chloroform (9mL) solution was slowly added 0.144g (0.9mmol) ( R )-5-bromo-1-penten-4-yn-3-ol, 0.2081g (3mmol) hydroxylamine hydrochloride aqueous solution 2mL, keep the system light yellow, after the dropwise addition, continue to react for 1h. After the reaction, extract with chloroform, combine the organic phases, dry over anhydrous sodium sulfate, filter, concentrate, and perform silica gel column chromatography (petroleum ether: ether, 5:1) to obtain 0.14 g of light yellow oily liquid (85% yield) . [α] D 20 = -20.8 (c = 1.1, CHCl 3 ); 1 H NMR (300 MHz, CDCl 3): δ 5.95 (ddd, J = 17.04, 10.11, 5.31 Hz 1H), 5.44-5.50 (m, 1H), 5.23-5.27 (m, 1H), 4.90-4.94 (m, 1H), 3.62 (m, 2H ), 2.58 (m, 2H), 2.11 (br 3H), 1.25–1.58 (m, 12H), 0.88 (t, J = 6.87 Hz 3H); 13 C NMR (75 MHz, CDCl 3 ): Δ136.41, 117.6...
Embodiment 3
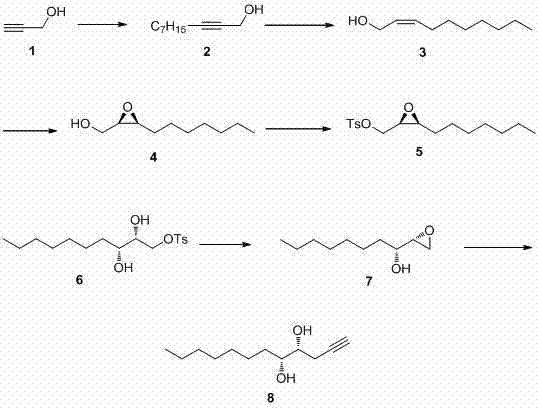
[0073] (4 R ,5 R )-dodecane-1-yne-4,5-diol is synthesized by
[0074] (1) Preparation of 2-decyn-1-alcohol (compound 2 )
[0075] Add 50mL tetrahydrofuran, 5.32mL (90mmol) propynyl alcohol, 39.5mL (225mmol) hexamethylphosphoric triamide to a 250mL reaction flask successively under nitrogen protection, and slowly add 72mL (180mmol) n-butyl at -78°C Lithium solution. After 0.5 h of dropwise addition, the temperature was raised to -30°C and stirred for 3 h, and 7.07 mL (45 mmol) of n-bromoheptane was added, stirred for 20 min, then raised to room temperature and continued to react for 21 h. After the reaction was completed, 30 mL of saturated ammonium chloride aqueous solution was added, extracted with ether (30 mL'3), and the organic phases were combined. The organic phase was washed with saturated aqueous sodium chloride solution, dried over anhydrous sodium sulfate, filtered, and concentrated. The crude product was subjected to silica gel (200-300 mesh) column chromatogra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com