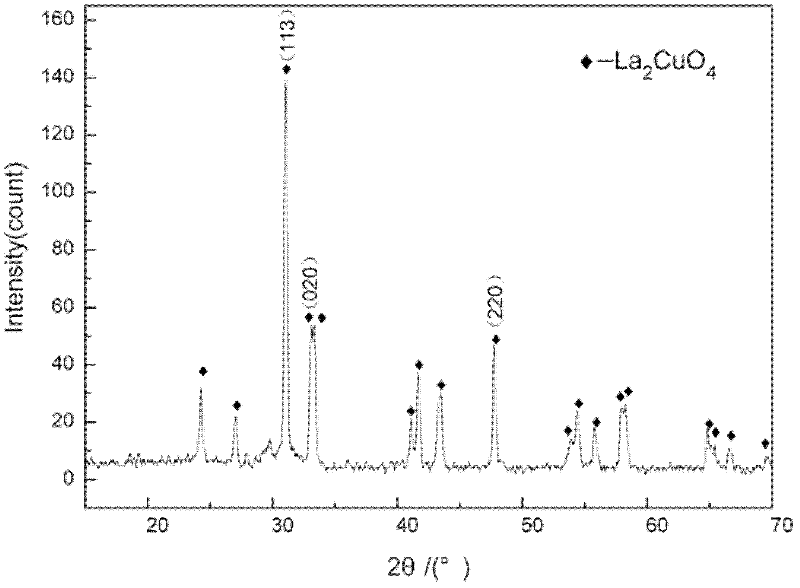
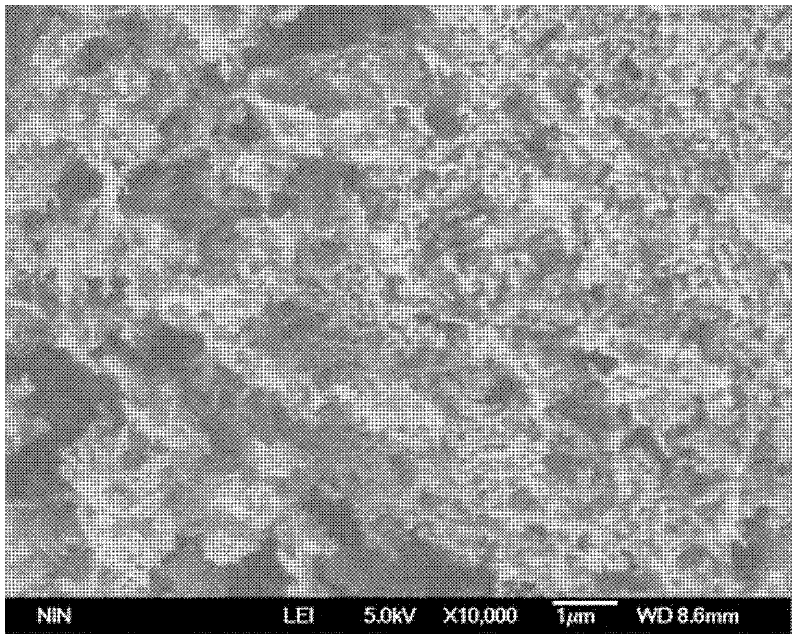
Co-precipitation method for preparing lanthanum cuprate (La2CuO4) powder
A co-precipitation method, lanthanum cuprate technology, applied in chemical instruments and methods, copper compounds, inorganic chemistry, etc., can solve the problems of high equipment requirements, high combustion temperature, fast reaction process, etc., and achieve narrow particle size distribution range, particle size The effect of controllable size and regular shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] 1) Analytical pure lanthanum nitrate (La(NO 3 ) 3 ·6H 2 O) be dissolved in 55ml distilled water and make the solution A that lanthanum nitrate concentration is 1.4mol / L;
[0020] 2) Press La 3+ : Cu 2+ =2:1 molar ratio adds analytically pure copper nitrate (Cu(NO 3 ) 3 ·3H 2 O) get solution B;
[0021] 3) Add polyethylene glycol (PEG-4000) of 3% by mass of solution B to solution B as a dispersant, stir well to make it mix uniformly to obtain solution C;
[0022] 4) Ammonia solution with a concentration of 3mol / L is injected into the burette, slowly dripped into the solution C, and the coprecipitation reaction is carried out. The titration rate is controlled at 4ml / min, and at the same time, it is continuously stirred to generate a precipitate immediately, and the pH value of the reaction system is controlled at 9. The precipitate was repeatedly washed with distilled water and absolute ethanol, and the precipitate after suction filtration was dried at 100°C to ob...
Embodiment 2
[0026] 1) Analytical pure lanthanum nitrate (La(NO 3 ) 3 ·6H 2 O) be dissolved in 70ml distilled water and make the solution A that lanthanum nitrate concentration is 2mol / L;
[0027] 2) Press La 3+ : Cu 2+ =2:1 molar ratio adds analytically pure copper nitrate (Cu(NO 3 ) 3 ·3H 2 O) get solution B;
[0028] 3) Add polyethylene glycol (PEG-4000) with 4% mass of solution B to solution B as a dispersant, stir well, and mix it uniformly to obtain solution C;
[0029] 4) Aqueous ammonia with a concentration of 4mol / L is injected into the burette, slowly dripped into the solution C, and the coprecipitation reaction is carried out. The titration rate is controlled at 5ml / min, and at the same time, it is continuously stirred to generate a precipitate immediately, and the pH value of the reaction system is controlled at 10. The precipitate was repeatedly washed with distilled water and absolute ethanol, and the precipitate after suction filtration was dried at 90°C to obtain the ...
Embodiment 3
[0032] 1) Analytical pure lanthanum nitrate (La(NO 3 ) 3 ·6H 2 O) be dissolved in 100ml distilled water and make lanthanum nitrate concentration be the solution A of 0.8mol / L;
[0033] 2) Press La 3+ : Cu 2+ =2:1 molar ratio adds analytically pure copper nitrate (Cu(NO 3 ) 3 ·3H 2 O) get solution B;
[0034] 3) Add polyethylene glycol (PEG-4000) of 2% by mass of solution B to solution B as a dispersant, stir well, and mix it uniformly to obtain solution C;
[0035] 4) Ammonia solution with a concentration of 5mol / L is injected into the burette, slowly dripped into the solution C, and the coprecipitation reaction is carried out. The titration rate is controlled at 3ml / min, and at the same time, it is constantly stirred to generate precipitation immediately, and the pH value of the reaction system is controlled at 7. The precipitate was repeatedly washed with distilled water and absolute ethanol, and the precipitate after suction filtration was dried at 70°C to obtain th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com