STAT3 small molecular selective inhibitor and preparation method and application thereof
A molecular selectivity and inhibitor technology, applied in the directions of active ingredients of heterocyclic compounds, drug combinations, organic chemistry, etc., can solve problems such as difficult medicinal use and poor bioavailability, and achieve good medical efficacy, strong practicability, and preparation. simple method effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Virtual Screening of Small Molecule Inhibitors Based on STAT3 Protein Structure
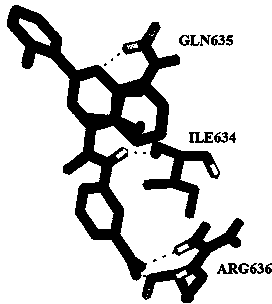
[0035] STAT3 organic small molecule inhibitor obtained through virtual screening. The protein crystal structure code 1BG1 was obtained from ProteinDataBank. The databases used in the virtual screening include Specs, Maybridge, these two databases include more than 400,000 organic compounds in total, these databases can be obtained from the ZINC database, and what is obtained is a three-dimensional model of the compound.
[0036] The molecular docking program Autod°k (version 4.2) and the virtual screening tool PyRx were used for virtual screening. In order to obtain a better screening structure, the chain B of the protein structure 1BG1 was removed from the structure, and processed by removing water, adding hydrogen, mixing non-polar hydrogen, and calculating GasteigerCharges. The docking area was set in the SH2 area of the protein, and the Gridbox setting Large enough to c...
Embodiment 2
[0037] The synthesis of embodiment 22-phenyl-6-chloroquinoline-4-carboxylic acid methyl ester
[0038] Mix 40mL of water and 90g of sodium sulfate in a 1000mL reaction flask, dissolve 9.92g of chloral in 20mL of water, and add to the reaction flask. Mix 5.51g of p-chloroaniline, 10mL of concentrated hydrochloric acid, and 70mL of water to form a uniform solution, and slowly drop it into the reaction flask. After the dropwise addition, 9.03g of hydroxylamine hydrochloride in 40mL aqueous solution was added dropwise, and the temperature was raised to 85°C for 2.5h after the dropwise addition, and then rapidly cooled to room temperature, a white solid was precipitated, suction filtered, washed with water, and dried to obtain 7.3g of the product N-(4- Synthesis of chlorophenyl)-2-oxime)acetamide 2. The reaction formula for this reaction is:
[0039]
[0040] The product is characterized, and the specific data are: 1 HNMR (300 MHz, DMSO): δ 12.19 (s, 1H), 10.29 (s, 1H), 7.75-7....
Embodiment 3
[0050] Synthesis of Example 32-(4-bromophenyl)-6-chloroquinoline-4-carboxylic acid methyl ester
[0051] The structural formula of 2-(4-bromophenyl)-6-chloroquinoline-4-carboxylic acid methyl ester is:
[0052]
[0053] Synthetic method is with the synthetic of 2-phenyl-6-chloroquinoline-4-carboxylate methyl ester among the embodiment 2, difference is to use 4-bromoacetophenone to replace the acetophenone in the example 2, obtain 2-(4 -Bromophenyl)-6-chloroquinoline-4-carboxylic acid, 2-(4-bromophenyl)-6-chloroquinoline-4-carboxylic acid reacts with methanol to obtain 2-(4-bromophenyl)- Methyl 6-chloroquinoline-4-carboxylate.
[0054] The product is characterized, and the specific data are: 1 HNMR (300MHz, DMSO): δ8.60(s, 1H), 8.47(s, 1H), 8.23(s, 2H), 8.10(s, 1H), 7.83(s, 1H), 7.46-1.62(m, 3H), 4.00(s, 3H); 13 CNMR (125MHz, DMSO): δ168.89, 157.95, 143.08, 138.15, 136.15, 132.46, 131.8, 131.72, 130.59, 130.22, 129.77, 125.43, 124.81, 123.11, 52.08; + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com