Method for preparing nickel oxide, magnesium oxide and silicon oxide products from laterite-nickel ore
A technology of lateritic nickel ore and silicon dioxide, which is applied in the direction of nickel oxide/nickel hydroxide, silicon dioxide, magnesium oxide, etc., can solve the problems of high equipment requirements, difficult control of operating conditions, and limitation of magnesium content, etc., and achieve technological process The effect of simple and high value-added comprehensive utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
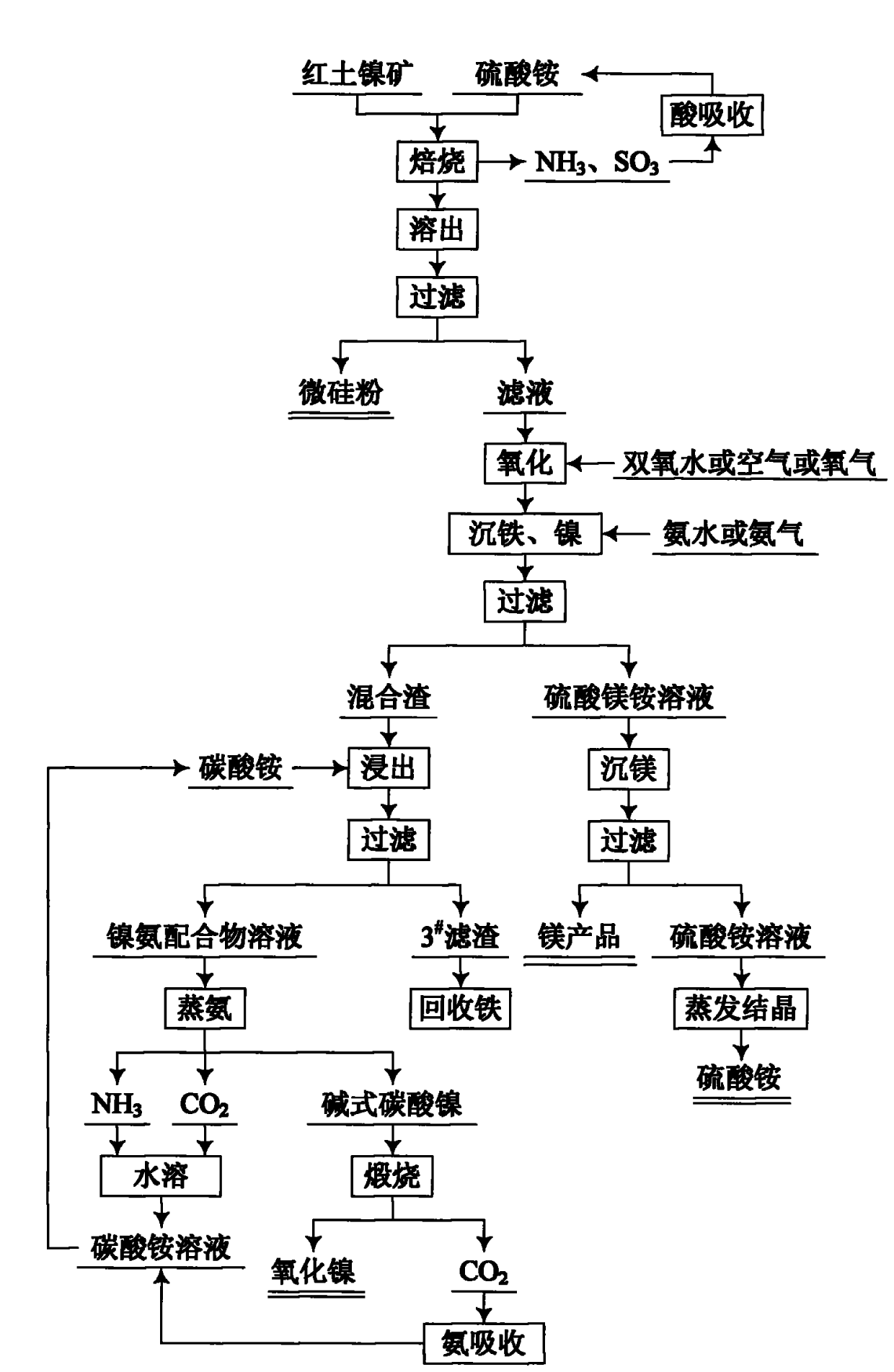
[0037] The composition of the laterite nickel ore used is: NiO 1.13%, SiO 2 41.74%, MgO 21.53%, Fe 2 o 3 18.82%, CaO 0.62%, Cr 2 o 3 0.56%, loss on ignition 11.63% other balance.
[0038] Mix the laterite nickel ore that has been crushed and ground to less than 80 μm and ammonium sulfate in a molar ratio of 1:3, dry and dehydrate at 150°C, and heat to 450°C for 5 hours. The ammonia and sulfur trioxide generated during the reaction process are dilute Sulfuric acid is absorbed to prepare ammonium sulfate solution, and ammonium sulfate crystals are prepared through evaporation, concentration and crystallization, which are used as raw materials for laterite nickel ore roasting. The roasted material was cooled, dissolved in 3 times the mass of water at 80°C for 40 minutes, and filtered to obtain the filtrate and 1# filter residue. The filtrate contains Ni 2+ , Fe 3+ , Fe 2+ , Mg 2+ , NH 4 + , SO 4 2- solution, and 1# filter residue as micro-silica fume product.
[...
Embodiment 2
[0044] The composition of the laterite nickel ore used is: NiO 1.73%, SiO 2 42.57%, MgO 20.31%, Fe 2 o 3 18.66%, Al 2 o 3 3.87%, CaO 0.68%, Cr 2 o 3 0.52%, other impurities 0.86%, loss on ignition 10.8%.
[0045] Mix the crushed and finely ground laterite nickel ore with ammonium sulfate at a molar ratio of 1:5, dry and dehydrate at 200°C, and heat to 400°C for 6 hours. The ammonia and sulfur trioxide generated during the reaction process are dilute Sulfuric acid absorption, preparation of ammonium sulfate solution, evaporation, concentration, and crystallization to prepare ammonium sulfate crystals, which are used as raw materials for laterite nickel ore roasting. The calcined material was cooled, dissolved in 5 times the mass of water at 85°C for 30 minutes, and filtered to obtain the filtrate and 1# filter residue. The filtrate contains Ni 2+ , Fe 3+ , Fe 2+ , Mg 2+ , NH 4 + , SO 4 2- solution, and the 1# filter residue is directly used as micro-silica fum...
Embodiment 3
[0051] The composition of the laterite nickel ore used is: NiO 2.52%, SiO 2 38.83%, MgO 22.42%, Fe 2 o 3 19.71%, Al 2 o 3 4.27%, CaO 0.53%, Cr 2 o 3 0.46%, other impurities 0.78%, loss on ignition 10.48%.
[0052] Mix the laterite nickel ore that has been crushed and ground to less than 80 μm and ammonium sulfate in a molar ratio of 1:4, dry and dehydrate at 150°C, and heat to 500°C for 4 hours. The ammonia and sulfur trioxide generated during the reaction process are dilute Sulfuric acid absorption, preparation of ammonium sulfate solution, evaporation, concentration, and crystallization to prepare ammonium sulfate crystals, which are used as raw materials for laterite nickel ore roasting. The roasted material was cooled, dissolved in 3 times the mass of water at 80°C for 40 minutes, and filtered to obtain the filtrate and 1# filter residue. The filtrate contains Ni 2+ , Fe 3+ , Fe 2+ , Mg 2+ , NH 4 + , SO 4 2- solution, and the 1# filter residue is directly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com